Synthesis and Application of Isobornyl methacrylate
Oct 19,2022
General description
Isborneol methacrylate is colorless transparent liquid. Isobornyl methacrylate is a kind of monomer that integrates hardness and flexibility. Due to its molecular structure characteristics, its polymer has excellent high light, fresh image, abrasion resistance, medium resistance and weather resistance, and its moisture absorption is significantly lower than MMA (methyl methacrylate). In addition, acrylic resin with IBOMA is soluble in polyester, alkyd and many volatile paint film forming substances.
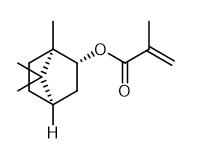
Fig. 1 The structure of Isobornyl methacrylate.
Synthetic routes
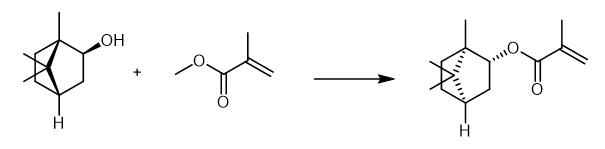
Fig. 2 The synthetic method 1 of Isobornyl methacrylate.
Add methyl methacrylate (1.49 mL, 14 mmol) to a mixture of sodium 2, 6-di-tert-butyl-4-methylphenolate (12.1 mg, 0.050 mmol), activated 5a? molecular sieves (powder, 400 mg), 4-acetamido-2, 2, 6, 6-tetramethylpiperidine 1-oxyl (4-acetamido-TEMPO) (0.42 mg, 0.0020 mmol) as a polymerization inhibitor and dimethyl sulfone (18.8 mg, 0.20 mmol) at 25°C for 3 min. Add alcohol (2.0 mmol) to the mixture at 25°C. Stir the mixture at 25°C for 0.5 h. Purify the resultant crude mixture by silica gel column chromatography (eluent: n-hexane and EtOAc). Chromatograph (eluent: n-hexane and EtOAc). 1H NMR (400 MHz, CDCl3) δ 0.85 (s, 3H), 0.86 (s, 3H), 1.02 (s, 3H), 1.07-1.21 (m, 2H), 1.57 (m, 1H), 1.67-1.88 (m, 4H), 1.93 (s, 3H), 4.71 (m, 1H), 5.52 (d, J = 1.4 Hz, 1H), 6.07 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 11.5, 18.4, 19.9, 20.1, 27.1, 33.7, 38.9, 45.1, 47.0, 48.9, 81.2, 125.0, 136.9, 166.9. IR (neat) 2955, 2879, 1717, 1638, 1455, 1327, 1298, 1163, 1054 cm-1. HRMS (FAB+) calcd for C14H22NaO2 [M+Na]+ 245.1517, found 245.1509 [1].
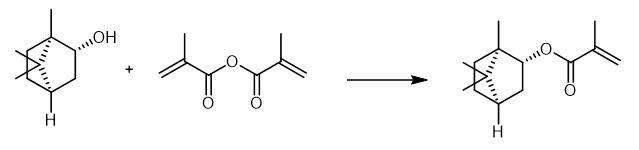
Fig. 3 The synthetic method 2 of Isobornyl methacrylate.
Dissolve 5g (1S,2S,4S)-(+)-isoborneol in 15 mL toluene. Add 4 mg (0.032 mmol) 4-methoxyphenol to the solution. After the addition of 0.4 g (3.24 mmol) 4-dimethylamino pyridine, add a solution of 10 g (64.8 mmol) methacrylic anhydride (MAA) in 10 mL toluene dropwise to the mixture. Heat the reaction mixture to 80 °C. Stir the reaction mixture for 16 hours. After cooling down to room temperature, wash the mixture with 50 mL 1M sodium bicarbonate solution four times. Wash the organic phase with 50 mL brine two times. Separate the organic phase. Remove the toluene by rotary evaporation followed by a fractional distillation under reduced pressure. 1H NMR (CDCl3, 300 MHz): δ(ppm) = 6.06 (dq, 1H, 1); 5.51 (dq, 1H, 2); 4.71 (m ,1H, 4); 1.93 (dd, 3H, 3); 1.87-1.5 (m, 5H, 6,7,8); 1.23-1.04 (m, 2H, 5); 1.012 (s, 3H, 9); 0.86 (s, 3H, 10); 0.85 (s, 3H, 11). IR (film): ν(cm-1) = 2954 (CH2), 2879 (CH2), 1715 (C=O) 1454 (CH2), 1157 and 1053 (C=O) [2].
Reactivity Ratios and Thermal Studies
Photocopolymerization of heterocyclic monomer namely, tetrahydrofurfuryl methacrylate with bulky bicyclic monomer, isobornyl methacrylate with different feed ratios was carried out in bulk with low concentration of an -hydroxyl ketone based photoinitiator. The ambient temperature photocopolymerization was carried out by using a UV-Visible lamp with fixed low intensity of 0.4mW cm(-2) for a period of 6min. The residual monomer remained in the polymerization process were determined by using gas chromatography. The reactivity ratio values for the two monomers were calculated from the copolymer composition data by using Fineman-Ross, Kelen-Tudos, Extended Kelen-Tudos and Mao-Huglin methods. Individually, as well as the average of all the methods revealed that the monomer reactivity ratios of tetrahydrofurfuryl methacrylate were higher than isobornyl methacrylate. The dyad sequence distribution and dyad sequence lengths were calculated using the Igarashi and Pyun method and the sequence length distribution for tetrahydrofurfuryl methacrylate was observed to be higher with an increase in its feed content. This supports the reactivity ratio studies that a higher monomer reactivity ratio value for tetrahydrofurfuryl methacrylate was observed as compared to its comonomer. The thermal studies showed that the glass transition temperatures of the copolymers increased with an increase in isobornyl methacrylate content [3].
Application
Synthesis of methacrylate copolymer
A methacrylate copolymer based on isobornyl methacrylate (IBMA) and methyl methacrylate (MMA) was synthesized in an aqueous suspension via free-radical polymerization. The potential of this copolymer as a heat-resistant optical polymer is also discussed. 1,1,3,3-tetramethylbutyl peroxy-2-ethyl hexanoate and n-octyl mercaptan were used as the initiator and chain transfer agents, respectively. The effect of IBMA on the properties of the copolymer was investigated. The composition of the copolymer was analyzed using H-1-NMR, and the heat resistance by measuring the glass transition temperature, which exhibited a linear dependency on the IBMA content in the copolymer. Variation of the chain transfer content used in the synthesis step was effective for the optimization of the copolymer for practical use [4].
A copolymer of 4-methoxybenzyl methacrylate and isobornyl methacrylate was synthesized by atom transfer radical polymerization. The structure of poly(4-methoxybenzyl methacrylate-co-isobornyl methacrylate) was confirmed by means of Fourier transform infrared, H-1-NMR, and C-13-NMR techniques. The molecular weight distribution values of the copolymer were determined with gel permeation chromatography. The number-average molecular weight and polydispersity index values of poly(4-methoxybenzyl methacrylate-co-isobornyl methacrylate) were found to be 12,500 and 1.5, respectively. The kinetics of the thermal degradation of the copolymer was investigated with thermogravimetric analysis at different heating rates. The activation energy values obtained with the Kissinger, Flynn-Wall-Ozawa, and Tang methods were determined to be 166.38, 167.54, and 167.47 kJ/mol, respectively. Different integral and differential methods were used, and the results were compared with these values. Doyle approximation was also used for comparing the experimental results to master plots. An analysis of the experimental results suggested that the reaction mechanism was an R, deceleration type in the conversion range studied [5].
References
[1] Ng J Q, Arima H, Mochizuki T, et al. Chemoselective Transesterification of Methyl (Meth) acrylates Catalyzed by Sodium (I) or Magnesium (II) Aryloxides[J]. ACS catalysis, 2020, 11(1): 199-207.
[2] Peters O, Ritter H. Chiral recognition of enantiomeric isobornyl methacrylate‐containing hydrogels with α‐cyclodextrin[J]. Polymer International, 2016, 65(2): 245-249.
[3] Rajdeo K S, Ponrathnam S, Pardeshi S, et al. Ambient temperature photocopolymerization of tetrahydrofurfuryl methacrylate and isobornyl methacrylate: reactivity ratios and thermal studies[J]. Journal of Macromolecular Science, Part A, 2015, 52(12): 982-991.
[4] Park S I, Lee S I, Hong S J, et al. Suspension Polymerization and Characterization of Transparent Poly (methyl methacrylate-co-isobornyl methacrylate)[J]. Macromolecular research, 2007, 15(5): 418-423.
[5] Kurt A,
- Related articles
- Related Qustion
Phloretin is used in cosmetics for its antioxidant, anti-inflammatory, whitening, sunscreen, antibacterial, and moisturizing effects.....
Oct 18,2022Biochemical EngineeringPolyhexamethylene guanidine hydrochloride (PHMG) is a new environment-friendly guanidine disinfectant.....
Oct 19,2022APIIsobornyl methacrylate
7534-94-3You may like
Isobornyl methacrylate manufacturers
- Isobornyl methacrylate
-

- $0.00 / 200Kg/Drum
- 2024-07-26
- CAS:7534-94-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1000mt
- Isobornyl methacrylate
-

- $0.00 / 1KG
- 2024-07-25
- CAS:7534-94-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Isobornyl methacrylate
-

- $0.00 / 200kg
- 2024-06-05
- CAS:7534-94-3
- Min. Order: 20kg
- Purity: 98.5%
- Supply Ability: 10 toms





