Synthesis and Application of Undecanedioic Acid
Oct 17,2022
General description
Undecanedioic acid is a kind of long chain dicarboxylic acid, which is a fine chemical product with important and extensive industrial applications. Long chain dicarboxylic acids refer to aliphatic dicarboxylic acids containing more than 10 (including 10) carbon atoms in the carbon chain. They and their derivatives are widely used in the production of special lubricants for aerospace and military industries, special materials for petroleum pipelines, automotive materials, high-grade plastic plasticizers, tire wear resistance agents, high-grade paint coatings, pharmaceuticals, pesticides and other fields, and eventually form a series of products with high added value. Its market demand is growing rapidly.

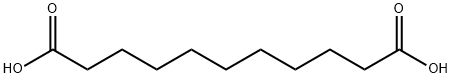
Fig. 1 The structure of Undecanedioic acid.
Synthetic routes

Fig. 2 The synthetic method 1 of Undecanedioic acid.
Dissolve periodic acid (11.4 g, 50 mmol) and CrO3 (23 mg, 0.23 mmol) in wet acetonitrile (75% CH3CN/water) to a volume of 114 mL (complete dissolution typically required 1-2 hours). Add H5IO6/CrO3 stock solution (13 mL, 2.5 equivalents of H5IO6 and 0.01 equivalent of CrO3) to a solution of 11-hydroxyundecanoic acid (470 mg) in wet acetonitrile (12 mL, 75% CH3CN/water) while maintaining the reaction temperature at 0-5 °C for 30 minutes. Perform aging of the mixture at 0 °C for 45 minutes. Quench the reaction by adding an aqueous solution of Na2HPO4 (0.7 g in 7 ml H2O). Add ethyl acetate (20 mL) to the mixture. Stir the mixture. Separate the organic layer. Saturate the aqueous phase with NaCl. Extract the aqueous phase with 10% MeOH-ethyl acetate (4 × 20 ml). Wash the combined organic layer with saline (15 ml), 5% NaHSO3 (15 mL) and saline (15 mL). Dry the organic layer over anhydrous Na2SO4. Concentrate the organic layer. Purify the product by recrystallization from ethyl acetate-hexane. 1H NMR (300 MHz, DMSO-d6) δ 2.17 (t, J = 6.6 Hz, 4H), 1.49-1.44 (m, 4H), 1.23 (m, 10H). 13C NMR (75 MHz, DMSO-d6) δ 174.90 (2C), 34.09 (2C), 29.23, 29.14 (2C), 28.98 (2C), 24.92 (2C) [1].

Fig. 3 The synthetic method 2 of Undecanedioic acid.
To a solution of α -nitro ketone (0.7 mmol) in MeOH (4.2 ml), 25 ml of 0.5 M Na2HPO4 in a 1 N of NaOH were added and the resulting mixture was heated at 70 °C for 1-4 h, then Oxone? (1.75 mmol) in water (3 ml) was added to the cold solution (r. t.). After 4 h the mixture was diluted with brine (10 ml), acidified to pH=2 with a 10% solution of HCl and extracted with EtOAc (3 x 20 ml). The combined organic layers were washed with brine (10 ml), dried over Na2SO4 and evaporated to yield pure dicarboxylic acid [2].

Fig. 4 The synthetic method 3 of Undecanedioic acid.
Maintain the reaction mixture of alkene derivative (1 mol), water (4 mol, 4 equiv.), THF (300 mL), PdCl2 (1.0 mmol, 0.1 mol%), ligand (4.0 mmol, 0.4 mol%), PTSA.H2O (8 mmol, 0.8 mol%), under 50 atm CO at 125 °C, 24 hours. Remove the precipitate by filtration. Concentrate the reaction solution and recrystallize for the first time at room temperature. Concentrate the mother liquor and recrystallize again in fridge (4 °C). Recrystallize the mother liquor one time more to obtain undecanedioic acid. 1H NMR (400 MHz, d6-DMSO) δ 11.97 (s, 2H), 2.25-2.15 (m, 4H), 1.52-1.43 (m, 4H), 1.30-1.20 (m, 10H). 13C NMR (101 MHz, d6-DMSO) δ 174.5, 33.7, 28.9, 28.8, 28.6, 24.6. HRMS (ESI): Calcd. for C11H19O4-: 215.1289, Found: 215.1283 [M-H]- [3].
Solvent-Dependent Conformational Polymorph Selectivity
Shi et al. report the polymorph nucleation of undecanedioic acid (UDA) having high solvent-dependent selectivity. Solvents with high hydrogen bond donating (HBD) ability preferred to produce form II, while form I was obtained from solvents with no HBD ability. Cooling experiments in a series of binary solvents with an HBD ability value from 0 to 86 were designed to affirm the strong correlation between solute-solvent interactions and polymorph formation verified by solution Fourier transform infrared (FTIR) spectroscopy and quantitative powder X-ray diffraction. Two crystal structures, evaluated as conformational dimorphs assisted by FTIR, solid-state NMR, and quantum chemistry calculations, were reported. Furthermore, the crystal structures suggest that UDA molecules from various solutions went through different conformation rearrangements resulting in two forms during nucleation. Meanwhile, we reveal that there is no direct connection between the solution chemistry of UDA in solvents with various HBD strengths and the corresponding forms. The results imply that the difficulty of desolvation, closely linked with solute-solvent hydrogen-bonding interaction, markedly affected the degree of conformation rearrangement and the nucleation outcome [4].
Application
Synthesis of novel even-odd nylons
A series of novel even-odd nylons were synthesized through step-heating melting-polycondensation of undecanedioic acid with various diamines. The synthesized polyamides were characterized comprehensively by means of elements analysis, intrinsic viscosity, Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR), Raman spectra, thermogravimetry analysis (TGA) and differential scanning analysis (DSC). The obtained products except nylons 4 11 and 2 11 have intrinsic average molecular weights ranging from 1.3 x 10(4) to 2.5 x 10(4). Nylons 4 11 and 2 11 have intrinsic average molecular weights of 8400 and 7200, respectively. The melting temperatures of this series of even-odd nylons decrease with declining the concentration of hydrogen bonds. Furthermore, the dynamic mechanical analysis (DMA) was performed for nylons 12 11, 10 11, 8 11 and 6 11. The glass transition temperatures of nylons 12 11, 10 11, 8 11 and 6 11 are in the range of 40.1-45.7degreesC [5].
A series of odd-odd polyamides were prepared through step-heating melt-polycondensation of undecanedioic acid with various diamines. The synthesized nylons were characterized comprehensively by means of elemental analysis, fourier-transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR), Raman spectra, intrinsic viscosity, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The intrinsic viscosities of the prepared polyamides except nylon 3 11 are 0.70 to 0.87 dlg(-1). The melting temperatures of the odd-odd nylons range from 182 to 209degreesC. In addition, the thermal mechanical properties of nylons 5 11, 7 11, 9 11 and 11 11 were analyzed using dynamic mechanical analysis (DMA). Nylon 3 11 could not undergo DMA due to its low molecular weight. The glass transition temperatures obtained from DMA are in the range 22-39degreesC [6].
References
[1] Jang H Y, Singha K, Kim H H, et al. Chemo-enzymatic synthesis of 11-hydr
- Related articles
- Related Qustion
Saccharin sodium is a kind of food additive and has no nutritional value to human body. When eating more, it will affect the normal secretion of digestive enzymes, reduce the absorption capacity of the small intestine.....
Oct 14,2022Diagnostic AgentsBenzalacetone is the organic compound described by the formula C10H10O.It has a white crystal appearance, commonly used as spices, mordant, galvanized brightener and organic synthesis reagents.....
Oct 17,2022Organic ChemistryUndecanedioic acid
1852-04-6You may like
Undecanedioic acid manufacturers
- Undecanedioic acid
-

- 2025-12-15
- CAS:1852-04-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Undecanedioic acid
-
- $1.00 / 1kg
- 2025-12-11
- CAS:1852-04-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Undecanedioic acid
-

- $0.00 / 1kg
- 2025-12-05
- CAS:1852-04-6
- Min. Order: 1kg
- Purity: 99%min
- Supply Ability: 20tons