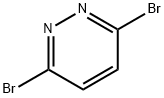
Synthesis and Precautions of 3,6-Dibromopyridazide
Oct 10,2022
Generally speaking
3,6-Dibromopyridazine is an important intermediate in organic synthesis. It is mainly used in pharmaceutical intermediates, organic synthesis, organic solvents, and can also be used in dye production, pesticide production and perfumery.
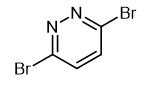
Fig. 1 The structure of 3,6-Dibromopyridazide.
Synthetic routes
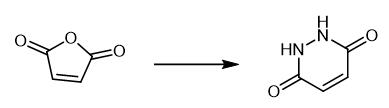
Fig. 2 Step 1 in the synthetic method 1 of 3,6-dibromopyridazine.
Add maleic anhydride (2.00 g) to a solution of hydrazine monohydrate (64% v/v, 1.2 mL, 16 mmol) in water (11 mL). Heat the resulting slurry to reflux in a preheated oil bath at which point the reaction becomes homogeneous. After 16 hours, cool the reaction mixture to room temperature. Dilute the reaction mixture with water (5 mL). A precipitate is formed. Isolate the material by filtration. Wash the material with water (3 × 5 mL) [1].
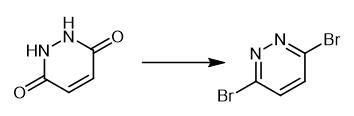
Fig. 2 Step 2 in the synthetic method 1 of 3,6-dibromopyridazine.
Charge a flask with maleic hydrazine (1.00 g) and freshly distilled POBr3 (5.73 g g, 20.0 mmol). Heat a homogeneous solution formed upon to 100°C in a preheated oil bath. After 3 hours, pour the reaction over a slurry of ice/water (~50 mL). Cool the mixture to 0°C. Adjust the pH to ~10 by addition of 10 M aqueous NaOH at 0°C. A precipitate is formed. Isolate the precipitate by filtration. Wash the precipitate with water (3 × 10 mL). 1H NMR (400 MHz, CDCl3) δ 7.52 (s, 2H) [1].
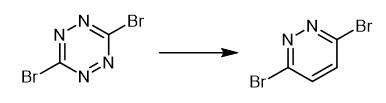
Fig. 3 The synthetic method 2 of 3,6-Dibromopyridazide.
Perform the reaction in a two-chamber reactor of reversed Y-tube type or H-tube type. Load the substrate part of the two-chamber reactor with 0.3 mmol of reactant and 1.0 ml of 1, 4-dioxane. Place 2 mmol of CaC2 and a solvent (0.6 ml) in the 2and part of the reactor. Add 4 mmol of water carefully to the carbide vessel. Seal the reactor thoroughly with a cup. Start the stirring carefully at small speeds (100 rpm) to avoid vigorous acetylene formation and undesirable transfer of water to the substrate part. Intensify the stirring gradually (upto 1400 rpm) when the release of acetylene almost stopped. Stir the reaction mixture at room temperature for the next 5.-.7 days. Collect the contents of the substrate part carefully using a syringe and evaporate the solvent. 1H NMR (400MHz,CDCl3) δ 7.53 (s, 2H). 13C NMR (101MHz,CDCl3) δ 147.7 (2C),133.5 (2CH). HRMS (ESI) Calcd.for C4H3Br2N2+ [M+H]+ 238.8637,found 238.8633. MP 95.-.97°C [2].
Hazard statement
1. Harmful if swallowed.
2. Causes skin irritation.
3. Causes serious eye irritation.
4. May cause respiratory irritation.
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Incident Response
1. If swallowed and uncomfortable: Call a POISON CENTER or doctor immediately.
2. IF ON SKIN: Wash with plenty of soap and water.
3. IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
4. In case of contact with eyes, rinse slowly and gently with water for several minutes. If contact lenses are present and easily accessible, remove contact lenses and continue rinsing.
5. If you feel unwell, call a poison control center or doctor.
6. If skin irritation occurs: Get medical advice/attention.
7. If eye irritation persists: Get medical advice/attention.
8. Take off soiled clothing and wash before reuse.
References
[1] Basistyi V S, Frederich J H. Pyridazine N-Oxides as Photoactivatable Surrogates for Reactive Oxygen Species[J]. Organic Letters, 2022, 24(10): 1907-1912.
[2] Ledovskaya M S, Polynski M V, Ananikov V P. One‐Pot and Two‐Chamber Methodologies for Using Acetylene Surrogates in the Synthesis of Pyridazines and Their D‐Labeled Derivatives[J]. Chemistry–An Asian Journal, 2021, 16(16): 2286-2297.
- Related articles
- Related Qustion
3,6-Dibromopyridazide
17973-86-3You may like
- Isothiazolinones: A Functional Biocides
Mar 12, 2025
- Guide to Polyethylene Terephthalate
Mar 12, 2025
- Pyrimidine: anticancer activity
Mar 12, 2025
3,6-Dibromopyridazide manufacturers
- 3,6-Dibromopyridazide
-

- $0.00 / 1KG
- 2022-01-04
- CAS:17973-86-3
- Min. Order: 1g
- Purity: 99%minHPLC
- Supply Ability: 100kgs/month
- 3,6-Dibromopyridazide
-
- $1.10 / 1g
- 2021-11-02
- CAS:17973-86-3
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min
- 3,6-Dibromopyridazide
-

- $8.80 / 1KG
- 2019-08-06
- CAS:17973-86-3
- Min. Order: 1KG
- Purity: 97%-99%
- Supply Ability: 100kg