Synthesis and Thermodynamic Study of N-(9-Fluorenylmethoxycarbonyloxy)succinimide
Jul 15,2022
General description
N-(9-Fluorenylmethoxycarbonyloxy)succinimide is a highly efficient selective reagent for the synthesis of hydroxyl amino acid derivatives and glycopeptides.

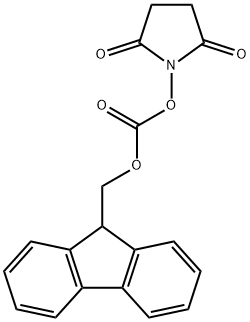
Fig. 1 The structure of N-(9-Fluorenylmethoxycarbonyloxy)succinimide.
Physicochemical property
N-(9-Fluorenylmethoxycarbonyloxy)succinimide is a white crystalline powder with a melting point of 147-151℃. It has a boiling point of 150-153°C and an estimated density of 1.3460 g/mL. It is soluble in dimethyl sulfoxide and dimethyl formamide.
Synthetic route
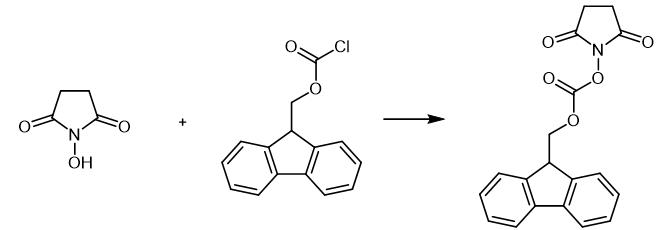
Fig. 2 The synthetic scheme of N-(9-Fluorenylmethoxycarbonyloxy)succinimide.
4 N-hydroxysuccinimide (450 mg, 3.90 mmol) and diisopropylamine (550 mL, 4 mmol) were dissolved in anhydrous dichloromethane (5 mL) under N2 at room temperature. The mixture was added drop wise to a solution of Fmoc chloride (1.007 g, 3.89 mmol) in anhydrous dichloromethane (5 mL) under N2 at 0°C with stirring over a period of 5 minutes. Upon completion, the flask was allowed to warm to room temperature, and the reaction mixture was stirred under N2 for 16 hours. The solution was filtered, andthe precipitate was washed with 20 mL dichloromethane. The filtrate and washings were combined and washed with, in order, 15 mL of 10% (w/v) citric acid in distilled water, 15 mL of 10% sodium bicarbonate (w/v) in distilled water, and 3 × 15 mL of distilled water. The organic layer was dried with anhydrous magnesium sulfate and filtered. The dichloromethane was removed in vacuo. Compound 6 (1.115 g, 85% yield) as a light yellow powder. The pure material was stored at 4°C under desiccation and used without further purification. 1H NMR (CDCl3, 500 MHz) δ 7.78 (d, 2H, J = 7.6 Hz), 7.63 (d, 2H, J = 7.6 Hz), 7.44 (t, 2H, J =7.5 Hz), 7.35 (dt, 2H, J = 7.5 Hz, 1.0 Hz), 4.58 (d,2H, J = 7.5 Hz), 4.35 (t, 1H, J = 7.4 Hz), 2.81 (bs, 4H); 13C NMR (CDCl3, 126 MHz) δ 168.6, 151.7, 142.6, 141.5, 128.4, 127.6, 125.4, 120.3, 73.1, 46.5, 25.6 ppm [1].
Equilibrium Solubility and Thermodynamic Studies
1. The equilibrium solubility of N-(9-fluorenylmethoxycarbonyloxy)succinimide in {dimethyl sulfoxide (DMSO), ethanol, ethylene glycol (EG), N,N-dimethylformamide (DMF)} plus water were determined by means of the isothermal equilibrium method at (283.15?328.15) K under atmosphere pressure. The measured values of N-(9-fluorenylmethoxycarbonyloxy)succinimide are positively increased with the increase of temperature in a certain cosolvent composition; however, that decreased when the content of water was gradually increasing and the largest solubility data was observed in pure DMF (0.04989 in mole fraction, 328.15 K). The mole fraction solubility in monosolvents ranked as DMF (9.693 × 10?3, 298.15 K) > DMSO (6.406 × 10?3, 298.15 K) > ethanol (8.237 × 10?4, 298.15 K) > EG (3.136 × 10?4, 298.15 K) > water (7.378 × 10?6). Under conditions of the same temperature and cosolvent composition, the solubility maximum of N-(9-fluorenylmethoxycarbonyloxy)succinimide was found in (DMF + water, 9.693 × 10?3 in mole fraction in DMF, 298.15K) than in the other aqueous mixtures system. The solid phase crystal samples of N-(9-fluorenylmethoxycarbonyloxy)succinimide were detected by XPRD which indicated clearly that there is no polymorphic transformation, solvate formation, or crystal transition for crystallized samples. General cosolvency models such as the Jouyban?Acree model, van’t Hoff?Jouyban?Acree model, and Apelblat?Jouyban?Acree model served to calculate and correlate the obtained solubility. Consequently, the difference between the measured values and calculated ones was quite small at evaluated temperatures so as the highest RAD (×10?2) and RMSD (×10?4) were 2.95 and 3.24, respectively[2].
2. Preferential solvation of N-(9-fluorenylmethoxycarbonyloxy)-succinimide (Fmoc-OSu) in mixtures of DMF (1) + water (2), ethanol (1) + water (2), ethylene glycol (EG, 1) + water (2) and DMSO (1) + water (2) was investigated by inverse Kirkwood–Buff integrals method. In ethanol-rich and intermediate contents, ethanol preferentially solvated Fmoc-OSu; and for the four aqueous co-solvent blends in water-rich composition regions, Fmoc-OSu was preferentially solvated by water. The solvent effect was described through modeling the Gibbs energy change of Fmoc-OSu solubility by linear solvation energy relationships. The main contribution to solubility variation of Fmoc-OSu was the solvent dipolaritypolarizability and solubility parameter. The dissolution and transfer enthalpy, entropy and Gibbs free energy were discussed in detail. Analysis outcomes of thermodynamic dissolution and enthalpy–entropy compensation demonstrated that two different mechanisms, enthalpy-driven and entropy-driven controlled the solubility variation of Fmoc-OSu in the DMF/EG/DMSO + water mixtures; and enthalpydriven, in the ethanol + water mixture [3].
References
[1] Robertson A W, Martinez-Farina C F, Smithen D A, et al. Eight-membered ring-containing jadomycins: implications for non-enzymatic natural products biosynthesis[J]. Journal of the American Chemical Society, 2015, 137(9): 3271-3275.
[2] Wu Y, Wu J, Cheng L, et al. The Dependence of Temperature and Composition on the Equilibrium Solubility and Thermodynamic Studies of Bioactive N-(9-Fluorenylmethoxycarbonyloxy) succinimide in Various Water–Cosolvent Mixtures[J]. Journal of Chemical & Engineering Data, 2018, 63(12): 4682-4688.
[3] Zhang F, Yang J, Liu H. Solubility of N-(9-fluorenylmethoxycarbonyloxy)-succinimide in several aqueous co-solvent solutions revisited: Solvent effect, preferential solvation and dissolution and transfer properties[J]. Journal of Molecular Liquids, 2021, 344: 117798.
- Related articles
- Related Qustion
Dexpanthenol (International Nomenclature of Cosmetic Ingredients (INCI) name panthenol) is the alcohol corresponding to pantothenic acid (the water-soluble vitamin B5).....
Jul 15,2022APIN-acetylneuraminic acid is a component of oligosaccharide chains of mucins, glycoproteins, and glycolipids that play an essential role in many physiological and immune recognition processes.....
Jul 15,2022APIN-(9-Fluorenylmethoxycarbonyloxy)succinimide
82911-69-1You may like
N-(9-Fluorenylmethoxycarbonyloxy)succinimide manufacturers
- N-(9-Fluorenylmethoxycarbonyloxy)succinimide
-

- $0.00 / 25Kg/Drum
- 2024-07-23
- CAS:82911-69-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year
- Fmoc-OSU
-

- $0.00/ kg
- 2024-07-23
- CAS:82911-69-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T
- Fmoc-Osu
-

- $20.00/ kg
- 2024-05-11
- CAS:82911-69-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 20