Synthesis, Detection and Application of Doxycycline Hydrochloride
Sep 9,2022
Generally speaking
Doxycycline hydrochloride, also known as doxycycline hydrochloride, is a light blue or yellow crystalline powder, odorless and bitter, hygroscopic, easily soluble in water and methanol, and slightly soluble in ethanol and acetone. This product has a broad antibacterial spectrum and is effective against Gram-positive cocci and negative Chemicalbook bacilli. The antibacterial effect is about 10 times stronger than that of tetracycline, and it is still effective against tetracycline-resistant bacteria. Mainly used for respiratory tract infections, chronic bronchitis, pneumonia and urinary tract infections, etc., but also for typhus, typhoid, and mycoplasma pneumonia.
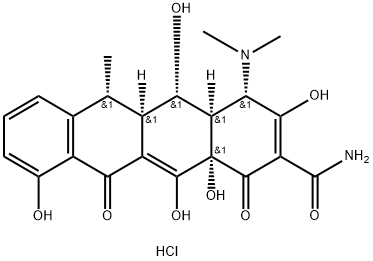
Fig. 1 The structure of Doxycycline hydrochloride.
Synthetic routes
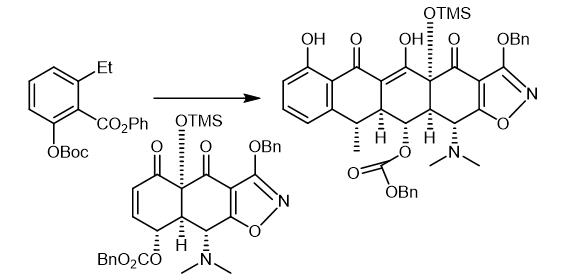
Fig. 2 The synthetic step 1 of Doxycycline hydrochloride.
A solution of n-butyllithium in hexanes (1.55 M, 155 pL, 0.241 mmol, 5.1 equiv) was added to a solution of N,N,N',N'-tetrainethylethylenediarnine (39.0 μL) 0.261 mmol, 5.5 equiv) and diisopropyl amine (34.0 μL, 0.249 mmol, 5.25 equiv) in tetrahydrofuran (1 mL) at -78 °C. The resulting mixture was stirred vigorously at -78 °C for 30 min whereupon a solution of the ester CDL-I-280 (73.0 mg, 0.213 mmol, 4.5 (1 equiv) in tetrahydrofuran (I mL) was added dropwise via cannula. The resulting deep red mixture was stirred vigorously at -78 °C for 75 min, then a solution ofthe silylcyclohexenone MGC26 (30.0 mg, 0.0474 mmol, 1.0 equiv) in tetrahydrofuran (1 mL) was added dropwise via cannula. The resulting light red mixture was allowed to warm slowly to 0 °C over 2 h, then was partitioned between an aqueous potassium phosphate buffer solution (pH 7.0, 0.2 M, 10 mL) and dichloromethane (10 mL). The organic phase was separated and the aqueous phase was further extracted with two 10-mL portions of dichloromethane. The organic phases were combined and dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, providing a yellow oil. The product was purified by preparatory HPLC on a Coulter Ultrasphere ODS column (10 μΜ, 250 x 10 mm, flow rate 3.5 mL/min, Solvent A: methanol, Solvent B: water) using an injection volume of 400 pL (methanol) and an isochratic elution of 10% B for 75 min. The peak eluting at 36-42 min was collected and concentrated, affording the Michael-Dieckmann addition product MGC27 (33.0 mg, 80%) as a light yellow solid [1].
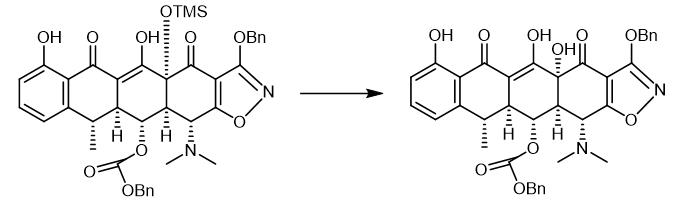
Fig. 3 The synthetic step 2 of Doxycycline hydrochloride.
Hydrofluoric acid (1.2 mL, 48% aqueous) was added to a polypropylene reaction vessel containing a solution ofthe Michael-Dieckmann addition product MGC27 (33.0 mg, 0.0375 mmol, 1.0 equiv) in acetonitrile (7.0 mL) at 23 °C. The resulting mixture was stirred vigorously at 23 °C for 60 h, then was poured into water (50 mL) containing K2HPO4 (7.0 g). The resulting mixture was extracted with ethyl acetate (3 x 20 mL). The organic phases were combined and dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate was concentrated, furnishing the pentacyclic phenol MGC28 as a yellow oil (25.0 mg, 99%). The product was used in the next step without further purification [1].
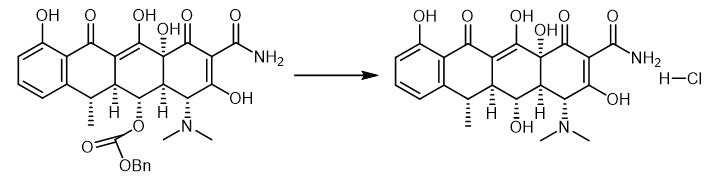
Fig. 4 The synthetic step 3 of Doxycycline hydrochloride.
Pd black (7.00 mg, 0.0657 mmol, 1.75 equiv) was added in one portion to a solution ofthe pentacyclic phenol MGC28 (25.0 mg, 0.0375 mmol, 1.0 equiv) in tetrahydrofuran-methanol (1:1, 2.0 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (I atm). The Pd catalyst was initially present as a fine dispersion, but aggregated into clumps within 5 min. The yellow heterogeneous mixture was stirred at 23 °C for 2 h, then was filtered through a plug of cotton. The filtrate was concentrated, affording a yellow oil (>95% doxycycline based on 1H NMR analysis). The product was purified by preparatory HPLC on a Phenomenex Polymerx* DVB* column (10 μΜ, 250 x 10 mm, flow rate 4.0 mL/min, Solvent A: methanol-0.005 N aq. HCl (1:4), Solvent B: acetonitrile) using an injection volume ofsolvent A (400 uL) containing oxalic acid (10 mg) and an isochratic elution of 5% B for 2 min, then a gradient elution of 5-50% B for 20 min. The peak eluting at 12-17 min was collected and concentrated, affording (-)- doxycycline hydrochloride as a yellow powder (16.2 mg, 90%), which was identical with natural (-)-doxycycline hydrochloride in all respects [1].
Detection Method
Doxycycline hydrochloride can enhance the chemiluminescence of potassium ferricyanide and luminol in alkaline medium. So a molecular imprinting-flow injection chemiluminescence method for the determination of doxycycline hydrochloride was established by using doxycycline hydrochloride-imprinted polymers as recognition material and potassium ferricyanide and luminol as detection system. Doxycycline hydrochloride-imprinted polymer was synthesized using methacrylic acid as functional monomer and ethylene glycol dimethacrylate as cross-linker. The linear range is 9.0 x 10-7-6.0 x 10-5 g.mL-1 and the detection limit is 3.2 x 10-7 g.mL-1. The relative standard deviation for 6.0 x 10-6 g.mL-1 of doxycycline hydrochloride was 3.5% (n = 9). This method has been successfully applied to the determination of doxycycline hydrochloride in tablets and in urine samples [2].
A flow injection with pulsed amperometric detection for determination of doxycycline or chlortetracycline in pharmaceutical formulations is described. Doxycycline or chlortetracycline were studied at a gold rotating disk electrode with cyclic voltammetry as a function of pH of supporting electrolyte solution. The optimized PAD waveform parameters were obtained with a flow injection system. The optimized pulsed conditions of doxycycline were 1150 mV (versus Ag/AgCl reference electrode) detection potential (E-det) for 220 ms (150 ms delay time and 70 ms integration time), 1500 mV (versus Ag/AgCl reference electrode) oxidation potential (E-oxd) for 70 ms oxidation time (t(oxd)) and 250 mV (versus Ag/AgCl reference electrode) reduction potentail (E-red) for 400 ms reactivation time (t(red)). The optimized pulsed conditions of chlortetracycline were 1050 mV (versus Ag/AgCl reference electrode) detection potential (E-det) for 300 ms (200 ms delay time and 100 ms integration time), 1300 mV (versus Ag/AgCl reference electrode) oxidation potential (E-oxd) for 70 ms oxidation time (t(oxd)) and 250 mV (versus Ag/AgCl reference electrode) reduction potentail (E-red) for 400 ms reactivation time (t(red)). The optimized PAD waveform was applied to the determination of doxycycline hydrochloride and chlortetracycline hydrochloride standard solution and in pharmaceutical formulations. The linear dynamic ranges of doxycycline hydrochloride and chlortetracycline hydrochloride were 1 muM-0.1 mM. The sensitivity of this method was found to be 23 muA/mM for doxycycline hydrochloride and 33.76 muA/mM for chlortetracycline hydrochloride.
- Related articles
- Related Qustion
- Doxycycline hydrochloride: A look at this broad-spectrum antibiotic Mar 7, 2025
Doxycycline hydrochloride is very versatile because of its antibacterial and anti-inflammatory properties.
A neuroprotective and nootropic drug Noopept increased the frequency of spontaneous calcium transients in neurons of CA1 radial layer in cultured rat hippocampal slices.....
Sep 8,2022API4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline can be used in new polyester materials.....
Sep 13,2022APIDoxycycline hydrochloride
10592-13-9You may like
Doxycycline hydrochloride manufacturers
- Doxycycline hydrochloride
-

- 2025-12-04
- CAS:10592-13-9
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Doxycycline hydrochloride
-

- $0.00 / 1kg
- 2025-12-04
- CAS:10592-13-9
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Doxycycline hydrochloride
-

- $100.00 / 50kg
- 2025-12-03
- CAS:10592-13-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton