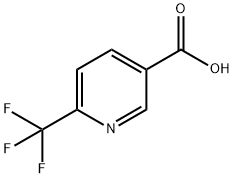
Synthesis of 6-(Trifluoromethyl)nicotinic acid
Aug 23,2022
General description
Trifluoromethylnicotinic acid is a very typical and important fluorine-containing pyridine compound. For example, 2-trifluoromethylnicotinic acid can be used as a calcium channel inhibitor, and 4-(Trifluoromethyl)nicotinic acid is a synthetic high-efficiency insecticide fluridine. The key intermediate of wormamide, 5-(Trifluoromethyl)nicotinic acid derivatives, has the potential to treat leukemia, and 6-(Trifluoromethyl)nicotinic acid is also the key intermediate of many fluorine-containing drugs.
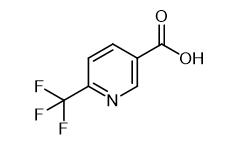
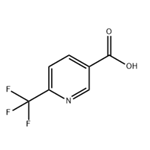
Fig. 1 The structure of 6-(Trifluoromethyl)nicotinic acid.
Physicochemical property
6-Trifluoromethylnicotinic acid is a white powder with a melting point of 193-197 °C. It has a boiling point of 259.3oC at 760 mmHg. Its density is 1.484 g/cm3. Its flash point, refractive index and vapor pressure are 110.6oC, 1.475 and 0.007mmHg at 25°C, respectively.
Synthetic routes
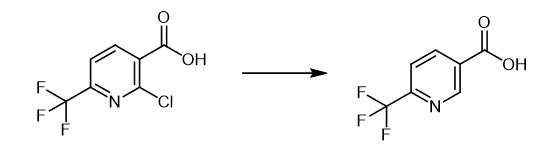
Fig. 2 The synthetic method 1 of 6-(Trifluoromethyl)nicotinic acid.
In 200 ml four-necked flask equipped with three-way cock, stirrer, and thermometer, 2-chloro-6-trifluoromethyl nicotinic acid 10 g (0.044 mol) and 50 ml methanol were charged, under stirring and then cooled in ice water, triethylamine 9.0 g (0.088 mol) was added dropwise. After replacing with nitrogen in the system, 1.0 g of 5%-Pd/C was introduced, Further hydrogen replaced by hydrogen in the system then it was attached to the balloon was filled. After holding at room temperature overnight with stirring, the reaction was followed by liquid chromatography,the raw materials of 2-chloro-6-trifluoromethyl nicotinic acid was disappeared mostly, and the reaction was stopped. After evaporation of the solvent methanol in the evaporator, 100 ml of water was added and dissolved the residue, to maintain the internal temperature at about 15. DEGREE CELSIUS. concentrated hydrochloric acid 9.3 g (0.089 mol) was added dropwise and then it was crystallized. After aging for about one hour at the same temperature,it was filtered by Nutsche, the cake was washed with 25 ml cold water. It was dried with 50 °C in automatic oven, 7.6 g of 6-trifluoromethyl nicotinic acid was obtained as an off-white powder. (crude yield 90.4%) [1].
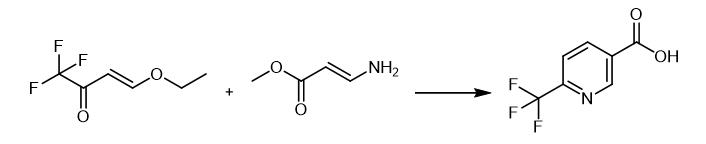
Fig. 3 The synthetic method 2 of 6-(Trifluoromethyl)nicotinic acid.
Methyl 3-amino acrylic acid 52g (0.086mol) (16.7 wt% methanol solution) and sodium methoxide (28 wt% methanol solution) 33.3g (0.173mol) was added below -5.DEGREE CELSIUS., Further 4-ethoxy-1,1,1-trifluoro-3-buten-2-one 15.6g (0.093mol) was added at -5 .DEGREE CELSIUS..The temperature of the reaction mixture was gradually raised,and the mixture was heated under reflux for 3 hours. To the reaction solution water 3 ml was added and the mixture was heated to reflux for an additional 30 minutes, it was concentrated under reduced pressure. Five times with 20 ml methylene chloride, after washing the reaction product was introduced in 50ml of water. After preparing concentrated hydrochloric acid with pH = 2, the crude product was filtered. After washing with water under repulping heating the crude product,filtration, washed with water, and then dried 6-trifluoromethyl nicotinic acid was obtained 7.03g (42.8% yield). (Melting point 170 ~ 176 .DEGREE CELSIUS.), 1H-NMR (400MHz, DMSO-d6) 8.04ppm, d, 1H, J = 8Hz, 8.54ppm, dd, 1H, J = 2Hz, 8Hz, 9.21ppm, d, 1H, J = 2Hz, 13.8ppm, br, 1H) [2].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Ando, Takayoshi. Preparation of 6-haloalkylnicotinic acids under mild conditions from 2-halo-6-haloalkylnicotinic acids[P]. Jpn. Kokai Tokkyo Koho, 2002201179, 2002.
[2] Yoneda T, Kanamori F, Kanbayashi S, et al. Preparation of 6-trifluoromethylnicotinic acids from aminovinyl compounds and 1,1,1-trifluorobuten-2-ones[P]. Jpn. Kokai Tokkyo Koho, 2001158774, 2001.
Diphenylphosphine oxide is an organophosphorus compound,it is an important intermediate in organic synthesis, widely used in the synthesis of various pesticides and chiral phosphine ligands.....
Aug 22,2022Organic ChemistryPF-07321332 (Nirmatrelvir) is a potent and orally active SARS-CoV?3C-like protease inhibitor.....
Aug 23,2022API6-(Trifluoromethyl)nicotinic acid
231291-22-8You may like
6-(Trifluoromethyl)nicotinic acid manufacturers
- 6-(Trifluoromethyl)nicotinic acid
-

- $0.00 / 1kg
- 2025-04-08
- CAS:231291-22-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- 6-(Trifluoromethyl)nicotinic acid
-
- $0.00 / 1KG
- 2025-04-04
- CAS:231291-22-8
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1Ton
- 6-(Trifluoromethyl)nicotinic acid
-

- $10.00 / 1kg
- 2025-04-01
- CAS:231291-22-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 ton