Synthesis of Surufatinib
Jan 5,2024
Synthesis of Surufatinib
Surufatinib is synthesised from dichloropyrimidine and hydroxyindole by substitution reaction. This process requires the participation of Surufatinib Amine.The specific synthesis steps are as follows:
Possible Approach to Surufatinib Amine
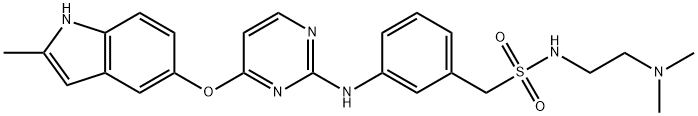
Sulfonyl chloride
358 was subjected to triethylamine with 2,2,2-trifluoroethanol
(TFE) as the solvent at ambient temperature followed by
hydrogenation to afford aniline 360. Next, exposure of 360 to
DBU under microwave conditions led to the formation of an
intermediate sulfene, which underwent nucleophilic attack by
commercial diamines in which diamine 361 could furnish the
desired sulfonamide 362. Although Golding and co-workers did
not explicitly describe a synthesis of 362, closely related analogs
were exemplified by this method using analogous substrates. It is
likely that a modified approach to 362 was used on process scale,
given the wide commercial availability of nitrobenzene 358 and
diamine 361.
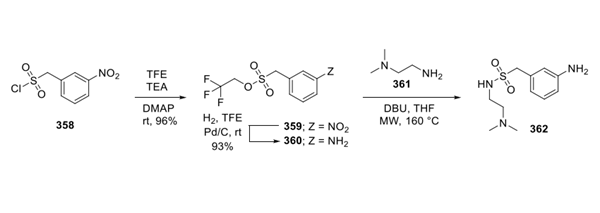
Synthesis of Surufatinib
Substitution of dichloropyrimidine 363 with hydroxyindole 364 gave rise to diaryl ether 365, which then participated in a second substitution reaction with amine 362 under acidic conditions. This was followed by exposure to aqueous base furnishing surufatinib in 50% yield from 365.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
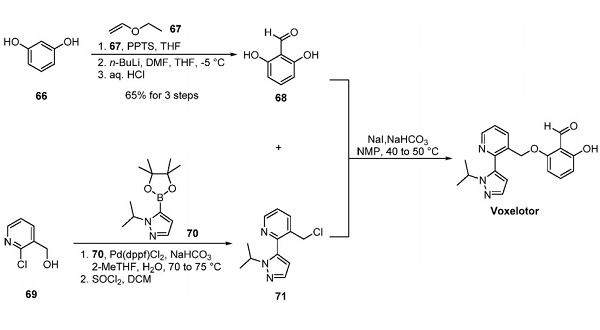
Nov 4,2025Biochemical EngineeringVoxelotor, also known as GBT440, is a relaxed-state (R-state) stabilizer of hemoglobin developed by Global Blood Therapeutics and approved by the USFDA for treating sickle cell disease.....
Jan 5,2024APISurufatinib
1308672-74-3You may like
- Sulfatinib
-

- $39.00 / 1mg
- 2025-12-10
- CAS:1308672-74-3
- Min. Order:
- Purity: 99.21%
- Supply Ability: 10g