Tamoxifen: Origins, Clinical Trials and Dose
Feb 28,2024
General Description
Tamoxifen, a pivotal drug in breast cancer treatment, originated from the mid-20th century's research on estrogen therapy. Clinical trials demonstrated its significant impact on reducing recurrence and mortality rates in early breast cancer patients, establishing 5 years of tamoxifen as the standard adjuvant therapy. Further studies explored extended tamoxifen treatment and compared it with aromatase inhibitors (AIs), revealing benefits and considerations for both options. The optimal dose of tamoxifen has been debated, with recent research indicating promising results for lower doses, such as 5 mg/day. This "baby-TAM" showed effectiveness in reducing neoplastic events and contralateral breast cancer, particularly in postmenopausal women. Further research is needed to confirm the role of lower doses in breast cancer therapy.

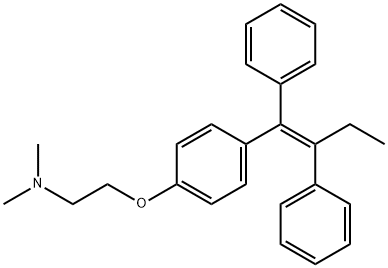
Figure 1. Tamoxifen
Origins
The origins of Tamoxifen can be traced back to the mid-20th century when the first endocrine therapy for breast cancer involved surgical oophorectomy. Subsequent research led to the discovery of ovarian estrogens and the development of long-acting synthetic estrogens like stilboestrol and triphenylethylenes in the 1940s. Stilboestrol was widely used for estrogen therapy until the emergence of tamoxifen three decades later. In the 1950s, the compound trimethylphenylethylene (M260) served as the foundation for the development of tamoxifen. The initial compound for tamoxifen was a mixture of cis and trans isomers. Through further research, the pure anti-estrogen cis isomer (ICI 47,699) was separated from the mixed isomer and eventually evolved into tamoxifen (ICI 46,474), which exhibited both estrogenic and anti-estrogenic properties. This crucial separation likely contributed to enhancing the success of tamoxifen as a significant milestone in breast cancer treatment. 1
Clinical trials
Tamoxifen, a selective estrogen receptor modulator (SERM), has revolutionized the treatment of early breast cancer and has significantly impacted clinical practice. Initially, clinical trials were conducted to assess the efficacy and toxicity of tamoxifen in comparison to the standard of no adjuvant therapy. The Early Breast Cancer Trials Collaborative Group (EBCTCG) reviewed multiple trials and found that tamoxifen significantly reduced recurrence and mortality rates in early breast cancer patients. The data from these trials led to the establishment of 5 years of tamoxifen as the standard adjuvant therapy. Further trials, such as the ATLAS trial, explored the extension of tamoxifen treatment to 10 years and demonstrated a significant reduction in recurrence and mortality with longer treatment duration. However, extended tamoxifen therapy was associated with small increases in pulmonary embolism and endometrial cancer, as well as a reduction in ischemic heart disease. In addition to tamoxifen, aromatase inhibitors (AIs) have been studied for the treatment of early breast cancer. The first adjuvant trial comparing postmenopausal women receiving either tamoxifen or anastrozole for 5 years showed that anastrozole was significantly superior in terms of relapse-free and overall survival. An overview analysis of trials comparing 5 years of tamoxifen with 5 years of AIs revealed a reduction in relapse for the first 5 years and a 10-year mortality advantage for AIs. The use of AIs in premenopausal women with concomitant ovarian suppression also showed a greater reduction in relapse, but the impact on mortality remains uncertain. Moreover, extending AI therapy beyond 7 or 8 years may not improve outcomes, and could lead to increased bone and cardiovascular toxicity. Future clinical trials may explore the potential of switching from AIs to tamoxifen for an additional 5 years in certain patient populations. In conclusion, tamoxifen has played a crucial role in the adjuvant treatment of early breast cancer, and ongoing research continues to refine the use of both tamoxifen and AIs to optimize outcomes for patients with breast cancer. 2
Dose
The optimal dose of tamoxifen in the treatment of breast cancer has been a subject of study and debate. Initial trials in advanced breast cancer explored daily doses ranging from 10 mg to 40 mg, with suggestions of a dose-response effect observed at higher doses. However, an overview of adjuvant trials indicated that the standard 20 mg dose was as effective as the higher 40 mg dose. Recent research has delved into lower doses of tamoxifen, such as 5 mg/day, with promising results. A study comparing tamoxifen 5 mg/day with placebo in women with breast intraepithelial neoplasia showed a significant reduction in neoplastic events with tamoxifen. Additionally, the 5 mg dose demonstrated a 75% reduction in contralateral breast cancer. This lower dose, referred to as 'baby-TAM,' was found to be more effective in postmenopausal women with specific baseline characteristics. Further investigations are warranted to establish the efficacy and positioning of low-dose tamoxifen compared to the standard 20 mg dose. Studies have also explored the use of mammographic density as a marker for tamoxifen responsiveness, with indications that lower doses may offer reduced toxicity without compromising effectiveness. While the potential for lower doses is promising, additional research is needed to solidify their role in breast cancer therapy. 2
Reference
1. Harper MJ, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966;212(5057):87.
2. Howell A, Howell SJ. Tamoxifen evolution. Br J Cancer. 2023;128(3):421-425.
- Related articles
- Related Qustion
- The introduction of Tamoxifen Oct 13, 2023
Tamoxifen is an antiestrogenic trans isomer of a substituted triphenyl ethylene. This drug is commonly used for treating pre- and perimenopausal women with estrogen receptor positive breast cancer.
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Dec 16,2024Biochemical EngineeringBis(cyclopentadienyl)zirconium dichloride is a highly reactive, selective, and stable catalyst used in polymerization reactions, including styrene and ethylene.....
Feb 28,2024APITamoxifen
10540-29-1You may like
- Tamoxifen
-

- $610.00 / 1Kg/Bag
- 2025-01-06
- CAS:10540-29-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1T
- Tamoxifen
-

- $0.00 / 2kg
- 2024-12-17
- CAS:10540-29-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- Tamoxifen citrate
-

- $1.00 / 10g
- 2024-11-01
- CAS:10540-29-1
- Min. Order: 10g
- Purity: 98%+
- Supply Ability: 100kgs