Tetrachloro-p-benzoquinone: A Notable Compound in Modern Chemistry
Sep 23,2024
Introduction
Tetrachloro-p-benzoquinone, also known as chloranil, is a significant compound in the field of organic chemistry. This compound, with the molecular formula C6Cl4O2, has found widespread applications across multiple industries, owing to its unique chemical properties. Initially synthesized in the 19th century, tetrachloro-p-benzoquinone continues to garner attention for its role in various chemical reactions, particularly in organic synthesis and as a reagent.

Figure 1 Characteristics of Tetrachloro-p-benzoquinone
Properties of Tetrachloro-p-benzoquinone
Tetrachloro-p-benzoquinone has a set of unique physical and chemical properties that distinguish it from other quinone derivatives. It appears as yellow to greenish-yellow crystals or powder, and it is soluble in organic solvents like benzene, ether, and acetone but is poorly soluble in water. This limited solubility in water is due to the hydrophobic nature of the chlorine atoms attached to the aromatic ring.
The melting point of tetrachloro-p-benzoquinone is approximately 290°C (554°F), and it sublimates readily at higher temperatures. Additionally, the compound is known for its ability to undergo reduction reactions, which convert it into tetrachlorohydroquinone. This redox behavior is a fundamental characteristic that allows tetrachloro-p-benzoquinone to function as a reagent in many organic reactions, particularly in oxidative processes.
In terms of chemical stability, tetrachloro-p-benzoquinone is relatively stable under normal conditions but can decompose when exposed to light or high temperatures. It can also react with strong reducing agents or bases, leading to the degradation of its quinone structure. Due to its reactivity, it must be handled with care in laboratory environments to prevent unwanted reactions or accidents.
Composition and Structure of Tetrachloro-p-benzoquinone
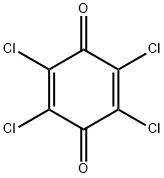
Tetrachloro-p-benzoquinone is composed of a six-membered benzene ring with alternating double bonds, making it an aromatic compound. The addition of four chlorine atoms and two carbonyl groups (C=O) to this structure converts it into a highly electron-deficient molecule. This electron deficiency is responsible for its high reactivity and ability to participate in electron-transfer processes, a key feature in its use as an oxidizing agent.
The structure of tetrachloro-p-benzoquinone can be visualized as a planar molecule, with the chlorine atoms attached at the 2, 3, 5, and 6 positions on the benzene ring, while the carbonyl groups occupy the 1 and 4 positions. This specific arrangement allows the compound to act as a versatile intermediate in various synthetic reactions, particularly those involving the transfer of electrons or functional group transformations.
From a molecular perspective, the electronegativity of the chlorine atoms increases the overall electron-withdrawing effect on the quinone core, enhancing its oxidizing ability. This feature is especially valuable in organic chemistry, where tetrachloro-p-benzoquinone is often used to introduce oxidative stress in substrates, driving specific transformations.
Applications of Tetrachloro-p-benzoquinone
Tetrachloro-p-benzoquinone has a wide range of applications across different chemical sectors, particularly in organic synthesis, dye production, and as a reagent in redox reactions.
Organic Synthesis: One of the most significant uses of tetrachloro-p-benzoquinone is in organic synthesis. It acts as a powerful oxidizing agent in reactions that require the removal of electrons from organic molecules. Chloranil is commonly employed in the synthesis of various aromatic compounds and as a catalyst in oxidative coupling reactions. Its reactivity makes it an essential tool in synthesizing complex molecules, including pharmaceuticals, agrochemicals, and specialty chemicals.
Dye and Pigment Industry: Tetrachloro-p-benzoquinone is also used in the production of certain dyes and pigments. Its ability to act as a precursor for the synthesis of other aromatic compounds has made it valuable in the development of colorants used in textiles, plastics, and printing. Specifically, chloranil has been used in the synthesis of indigo dyes and other compounds that provide vibrant colors in various materials.
Electronics and Conductive Polymers: Another emerging application of tetrachloro-p-benzoquinone is in the field of electronics and conductive polymers. Its electron-deficient structure allows it to interact with conjugated polymers, improving their conductivity and making it useful in the production of materials for electronic devices. Research into the use of tetrachloro-p-benzoquinone in organic semiconductors is ongoing, with the potential for it to contribute to advances in organic electronics.
Redox Reactions and Analytical Chemistry: In analytical chemistry, tetrachloro-p-benzoquinone is often used as a reagent in redox titrations and as an electron acceptor in various assays. Its well-defined redox potential allows for precise measurements in analytical procedures, making it an important tool in laboratories.
Storage and Handling of Tetrachloro-p-benzoquinone
Proper storage and handling of tetrachloro-p-benzoquinone are crucial to ensure safety and maintain the compound's stability. Due to its reactivity and potential to decompose under certain conditions, it is important to store tetrachloro-p-benzoquinone in a cool, dry place, away from direct sunlight and sources of heat. The compound should be kept in tightly sealed containers made from materials resistant to chemical reactions, such as glass or high-grade plastic.
When handling tetrachloro-p-benzoquinone, it is recommended to use personal protective equipment (PPE), including gloves, safety goggles, and protective clothing, to avoid skin contact or inhalation of dust. Adequate ventilation should be provided in workspaces where the compound is used, as inhalation of chloranil dust can be hazardous to health.
In case of a spill, it is essential to clean the area immediately while minimizing exposure. Chloranil should not be disposed of in regular waste systems due to its potential environmental impact. Instead, disposal should follow local regulations for hazardous chemicals, with appropriate measures taken to neutralize the compound before disposal.
Conclusion
Tetrachloro-p-benzoquinone, or chloranil, is a compound with significant importance in modern chemistry. Its unique properties, particularly its strong oxidizing ability and reactivity, have made it indispensable in various chemical applications, from organic synthesis to the production of dyes and pigments. As research continues to explore new uses for this compound, particularly in the field of electronics, its relevance in the chemical industry is likely to grow.
![]() Reference
Reference
[1] Plá F P, Palou J, Valero R, et al. Kinetics and mechanism of the addition of triphenylphosphoniocyclopentadienide to tetrachloro-p-benzoquinone[J]. Journal of the Chemical Society, Perkin Transactions 2, 1991 (12): 1925-1932.
[2] Hagen K, Hedberg K. The molecular structure of gaseous tetrachloro-p-benzoquinone and tetrachloro-o-benzoquinone by electron diffraction[J]. Journal of Molecular Structure, 1978, 49(2): 351-360.
- Related articles
- Related Qustion
- Application of Chloranil Apr 13, 2022
Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant. Solubility: insol
Sodium thiosulfate is a chemical compound with a diverse range of applications that highlight its importance in various industries.....
Sep 23,2024APIEthylene glycol diacetate is a compound with wide industrial application value. It is a diester derived from ethylene glycol and acetic acid.....
Sep 23,2024APIChloranil
118-75-2You may like
- Effect of Kinetin on Plant Growth
Sep 27, 2024
- Enrofloxacin: Mechanism of Action and Pharmacokinetics
Sep 27, 2024
- Poly(hexamethylenebiguanide)hydroch....
Sep 26, 2024