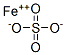The Comprehensive Guide to Ferrous Sulfate Monohydrate: Synthesis, Composition, and Applications
May 21,2024
Introduction
Ferrous sulfate monohydrate, a derivative of iron sulfate, is a chemical compound that plays a crucial role in various industrial and environmental processes. Known scientifically as FeSO4·H2O, this compound is characterized by its high solubility in water and its significant utility across multiple sectors. It is integral to numerous applications ranging from agriculture to medicine, contributing to its widespread use and recognition in the scientific community. The solubility of ferrous sulfate monohydrate in water makes it an excellent choice for formulations that require the rapid assimilation of iron, providing effective solutions in water treatment and nutrient supplementation. As a cornerstone in the production of other iron compounds, its importance extends into the realms of manufacturing and beyond, underlining its versatility and essential role in modern chemistry[1].

Figure 1 Characteristics of Ferrous Sulfate monohydrate
Synthesis of Ferrous Sulfate Monohydrate
The production of ferrous sulfate monohydrate predominantly involves the reaction of sulfuric acid with iron, or by oxidizing pyrite (FeS2). In industrial settings, it is commonly synthesized through the oxidation of ferrous sulfide or the direct reaction of iron with dilute sulfuric acid in an aqueous environment. This process not only yields ferrous sulfate monohydrate but also can generate by-products like hydrogen gas, depending on the specific conditions of the reaction.
Main Components
The primary component of ferrous sulfate monohydrate is iron(II) sulfate, with the chemical formula FeSO4. This compound also contains one molecule of water (H2O) per molecule of iron sulfate, contributing to its designation as a monohydrate. It typically appears as a blue-green, crystalline solid that is both odorless and water-soluble, making it highly effective for use in solution form. The monohydrate form ensures stability and ease of handling, which is crucial for its application in diverse industrial processes. Its crystal structure allows for effective dissolution and interaction in aqueous solutions, enhancing its reactivity and effectiveness in chemical reactions. Additionally, the presence of the water molecule in its structure helps in moderating reactions and facilitating controlled release of iron ions when used as a supplement or in agricultural applications, maximizing its efficacy and utility.
Applications of Ferrous Sulfate Monohydrate
Ferrous sulfate monohydrate finds extensive applications across various fields. In agriculture, it is commonly used as a soil amendment to correct iron deficiency in plants, which can cause chlorosis and reduce crop yield. Additionally, in the water treatment industry, it serves as a coagulant to remove impurities from water, aiding in the production of clean drinking water.
Another significant application is in the field of medicine, where it is used as a dietary supplement to treat iron-deficiency anemia. Moreover, ferrous sulfate monohydrate is employed in the dyeing industry for the production of iron salts, which are integral to the manufacturing of inks and dyes[2].
Storage Methods
Storing ferrous sulfate monohydrate requires specific conditions to maintain its chemical integrity and prevent degradation. It should be kept in a cool, dry place away from moisture and heat. The storage area should be well-ventilated to prevent the accumulation of any gases that might be released from the compound over time. Additionally, it should be stored in a container made of materials that do not react with ferrous sulfate, such as plastic or glass, to avoid contamination and ensure safety.
Conclusion
Ferrous sulfate monohydrate is a versatile compound with wide-ranging applications that underscore its importance in both industrial and environmental contexts. Its ability to address iron deficiency in agriculture, purify water, and treat anemia highlights its multifaceted utility. Understanding its synthesis, composition, and proper storage methods is essential for professionals dealing with chemicals to effectively utilize this compound and harness its full potential. As research and technology advance, the applications and efficiency of ferrous sulfate monohydrate are expected to expand, reinforcing its status as a critical chemical in numerous sectors.
![]() References
References
[1]Elgersma F, Witkamp G J, Van Rosmalen G M. Incorporation of zinc in ferrous sulfate monohydrate[J]. Hydrometallurgy, 1993, 33(3): 301-311.
[2]Meusburger J M, Ende M, Talla D, et al. Transformation mechanism of the pressure-induced C2/c-to-P1 transition in ferrous sulfate monohydrate single crystals[J]. Journal of Solid State Chemistry, 2019, 277: 240-252.
- Related articles
- Related Qustion
- Application of Ferrous sulfate May 24, 2022
Ferrous sulfate appears as a greenish or yellow-brown crystalline solid. Density 15.0 lb /gal. Melts at 64°C and loses the seven waters of hydration at 90°C.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering1,3-Benzodioxole, is a heterocyclic organic compound that is increasingly significant in various chemical applications.....
Nov 21,2024APIFerrous sulfate
7720-78-7You may like
- Ferrous sulfate
-
- 2025-12-13
- CAS:7720-78-7
- Min. Order:
- Purity: 0.99
- Supply Ability:
- FERROUS SULFATE
-

- $10.00 / 1kg
- 2025-12-11
- CAS:7720-78-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Ferrous Sulfate
-

- $650.00/ ton
- 2025-12-08
- CAS:7720-78-7
- Min. Order: 1ton
- Purity: 86%
- Supply Ability: 1000 tons