The configuration of pentaerythritol
May 31,2022
Introduxtion
Pentaerythritol, or tetramethylolmethane, is the tetrahydric alcohol and when pure is a colorless crystalline material melting without decomposition at 263°C. It can be sublimed under reduced pressure [1]. It is soluble in about 18 parts of water at 15"C., and can be recrystallized readily from hot water. It is also soluble to some extent in alcohol, acetone, dioxane, pyridine, and liquid ammonia. Pentaerythritol was first prepared by Tollens and Wigand[2] in 1891 by the action of formaldehyde on acetaldehyde in the presence of lime. Many modifications of the reaction have been reported in the patent literature, but essentially the same reaction is used today for the commercial production of pentaerythritol. A process in which caustic soda replaces the lime is also in operation commercially. The reaction takes place in one operation, but it can be represented as taking place in two stages:
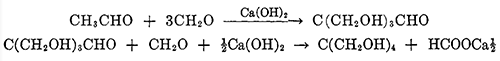
Pentaerythritol has been manufactured in large quantities during the last few years for conversion to its explosive tetranitrate. It has also a large potential market as a component in resinous composition.
Configuration
Early work on x-ray examinations of the crystal structure of pentaerythritol by Mark and Weissenberg [3] and Huggins and Hendricks [4] seemed to point to a pyramidal grouping of the four CHzOH groups around the central carbon atomin place of the generally accepted tetrahedral distribution, and work on the external symmetry of the crystals by Westenbrink and van Melle [5] and by Giebe and Scheibe [6] was supposed to confirm this. However Mark and Weissenberg, and Huggins and Hendricks, accepted the class CdV originally given by Martin [7], being unaware that Haga and Jaeger [8] had shown by means of a Laue photograph that the planes of symmetry supposed to intersect in the tetrad axis were non-existent. The C4 class was therefore unacceptable and various papers on this point followed.
Bromides
The pentaerythrityl bromides are probably the most important halides of pentaerythritol, as they are employed most frequently as starting products for the preparation of the other pentaerythritol derivatives. Beyaert and Hansens[9]have reported a convenient method for the preparation of pentaerythrityl mono- and di-bromides, involving the reaction of pentaerythritol and hydrogen bromide in glacial acetic acid followed by saponification of the bromoacetates formed. With varying proportions of hydrogen bromide a 65 percent yield of the monobromide together with 16 per cent of the dibromide, and an 80 percent yield of the dibrornide together with 18.5 percent of the tribromide, were obtained. The monobromide has also been isolated by fractional distillation at 1 mm. of the mother liquors from the preparation of the dibromide [10]. Barbiere and Matti [11] have prepared a mixture of the mono- and di-bromides by reaction of pentaerythritol with aqueous hydrobromic acid at 120°C. Zelinski [12] recorded the preparation of the dibromide by heating pentaerythritol and aqueous hydrobromic acid together at 125°C. however, Bincer and Hess[13] found that by this method there is also formed a considerable proportion of the tribromide.
Iodides
Pentaerythrityl monoiodide has only been prepared as a by-product ; it was isolated by Govaert and Beyaert [9]from the mother liquors from the prepara[1]tion of the diiodide, by fractional distillation at 1 mm. The diiodide has been prepared by Tollens and Wigand [10]by the reaction of pentaerythritol with hydriodic acid and red phosphorus at 170-180°C. also by Bincer and Hess [11], who obtained improved yields (45 per cent) by a similar method. By treatment of pentaerythritol with the same reagent at 19O"C., Tollens and Wigand[4]and Rave and Tollens [12] obtained the triiodide, together with a little tetraiodide. The tetraiodide is, however, best prepared by the action of sodium iodide on the tetrabromide in methyl ethyl ketone solution, as reported by Backer and Schurink [13]. The diiodide has also been obtained by Backer and Keuning [14] by the reaction of hydriodic acid with 2,6-dioxaspiro[3.3]heptane (VIII).
Reference
1 FILBERT, W. F. (to E. I. du Pont de Nemours & Co.): U. S. patent 2,358,697 (1944).
2 TOLLENS, B.AND WIGAND, P.: Ann. 266, 316 (1891).
3 MARK, H., AND WEISSENBERG, K.: Z. Physik 17, 301 (1923).
4 HUGGINS, &I. L., AND HENDRICKS, S. B. : J. Am. Chem. SOC. 48,164 (1926).
5 WESTENBRINK, H. G., AND MELLE, F. A. VAN: Z. Krist. 84, 548 (1926).
6 GIEBE! E., AND SCHEIBE, A.: Z. Physik 33, 760 (1925).
7 MARTIN, J.: Neues Jahrb. Mineral. Geol. 7, 1 (1890).
8 HAGA, H., AND JAEGER, F. 11.: Proc. Akad. Wetenschappen Amsterdam 18, 1350
9 GOVAERT, F., AXD BEYAERT, hl.: Xatuurw. Tijdschr. (Belg.) 21, 29 (1939); Chem. Abstracts 33, 9284 (1939).
10 BARBIERE, J., AND XIATTI, J.: Bull. Soc. chim. [5] 6, 1565 (1938).
11 ZELINSRI, N. D.: Ber. 46, 160 (1913); J. Russ. Phys. Chem. SOC. 44, 1870, 1873 (1912).
12 BINCER, H., AND HESS, K.: Ber. 61B, 537 (1928)
13 RAVE, P., AND TOLLENS, B.: Ann. 276, 58 (1893).
14 SCHURINK, H. B.: Organic Syntheses, Collective Vol. 11, p. 476. Chapman and Hall, Ltd., London (1943)
- Related articles
- Related Qustion
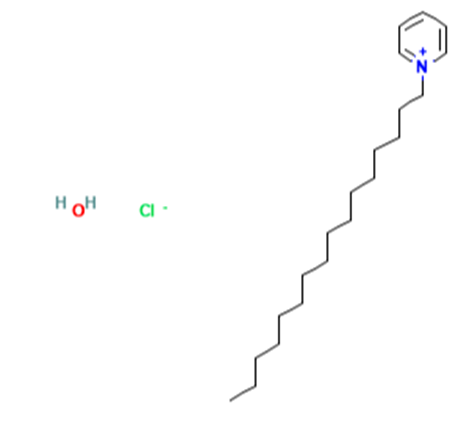
Cetylpyridinium chloride monohydrate is a hydrate. It contains a cetylpyridinium chloride. Cetylpyridinium Chloride is the chloride salt form of cetylpyridinium, a quaternary ammonium with broad-spect....
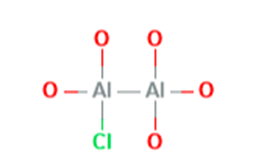
May 25,2022Organic Synthesis IntermediateAluminum chlorohydrate is a water soluble aluminum complex which contains several oligomeric polycations of aluminum. In acidic or neutral conditions, aluminum monomer Al3+ do form complexes with H2O....
May 31,2022DrugsPentaerythritol
115-77-5You may like