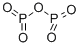The important application of phosphorus pentoxide
Aug 17,2022
Introduction
Phosphorus pentoxide is an acid anhydride that is obtained from phosphoric acid. It is highly hygroscopic and is, therefore, used as a dehydrating agent and as a desiccant. Phosphorus Pentoxide is a chemical compound whose empirical formula is P2O5 and whose molecular formula is P4O10[1].

Picture 1 Phosphorus pentoxide powders
Phosphorus pentoxide production system
The utility model discloses a phosphorus pentoxide production system, and belongs to the technical field of phosphorus chemical industry[1]. The system comprises a phosphorus storage tank, an overhead tank, a plurality of combustion furnaces, and a heat recovery structure, wherein the overhead tank is connected with the phosphorus storage tank; the plurality of combustion furnaces are arranged in parallel and are connected with the overhead tank through a phosphorus conveying pipeline; the heat recovery structure is arranged on combustion chambers and consists of at least one hot water bag; insulating jackets are arranged on the overhead tank and the phosphorus conveying pipeline; each combustion furnace comprises a combustion chamber and a settling chamber; the hot water bags are sequentially connected in series and then form a first circulating loop together with a hot water storage tank through a pipeline; and the insulating jackets and the hot water storage tank form a second circulating loop through a pipeline. According to the system, heat generated when yellow phosphorus burns is used for fusing the yellow phosphorus, so that energy sources are saved, and the production cost is reduced.
Application in 1, 5-benzodiazepines
In this report, a new method for the synthesis of 1, 5-bezodiazepines on a solid surface under microwave irradiation is described[2]. It is found that alumina (acidic)-supported phosphorus pentoxide under solvent-free conditions was capable of producing high yields of 2,3-dihydro-2,2,4-trimethyl-1H-1,5-benzodiazepine by condensation of o-phenylenediamine with acetone under mild reaction conditions in 85% yields under microwave irradiation. The same process was successfully extended to other 1,5-benzodiazepine derivatives. Acetophenone in the presence of mixture of acidic alumina/ phosphorus pentoxide with o-phenylenediamine afforded 2,3-dihydro-2-methyl-2,4-diphenyl-1H-1,5-bezodiazepine in 79 % yield. The other derivatives of acetophenones also react with o-pheneylenediamine in the presence of a mixture of acidic alumina/ phosphorus pentoxide under microwave irradiation, to give the desired compounds in moderate yields. The reaction also proceeds with good yield with cycloheptanone as a cyclic ketone to afford the desired product in moderate yield.
The condensation of unsymmetrical dialkyl ketones with o-phenylenediamine in the presence of this reagent, gave unknown products. This solvent-free method has operationally simple procedure. A five mmol of the o-phenylenediamine (finely ground) was added to a mixture of acidic alumina (Al 2 O 3 , acidic, 2-3 g, grinded in a mortar and pestle) and phosphorus pentoxide (0.5 g , 35 mmol). The ketone (10 mmol) was added to this mixture and the mixture was irradiated by microwave for 1 min using 720 W (A kitchen-type microwave was used in all experiments). The reaction mixture was grinded in a mortar and pestle until a fine, homogeneous, powder is obtained. Homogeneous mixture was washed with n-hexane (200 mL), and the filtrate was dried (CaCl 2), and the evaporated to give the crude products. Pure product was obtained by recrystallization from n-hexane in 73-85 % yields. In summary, simple work-up, low consumption of solvent, fast reaction rates, mild reaction condition, and good yields make this method an attractive and a useful contribution to present methodologies.
Application in phosphate monomer synthesis
A 100 mL round bottom vessel in an ice bath was charged with 50 mL of cold acetone. Phosphorus pentoxide (4.82 mmol) was added and the slurry was vigorously stirred with a magnetic bar. With an addition funnel, 29 mmol from HPMA synthesized by cyclohexane azeotrope method was added slowly during 1 h. Next, the ice bath was removed and the reaction was conducted at room temperature for 5 h. The product was filtered, 0.006 g of 2,6-di-tert-butyl-4-methyl phenol added and acetone evaporated in a rotatory evaporator[3]
Reference
1 WU HONGTAO; ZHANG SHUNQIN; ZHANG YONG, CN203976405U)
2 Kaboudin B, Navaee K. Alumina/phosphorus pentoxide (APP) as an efficient reagent for the synthesis of 1, 5-benzodiazepines under microwave irradiation[J]. Heterocycles, 2001, 55(8): 1443-1446.
3 Ogliari F A, da Silva E O, da Silveira Lima G, et al. Synthesis of phosphate monomers and bonding to dentin: esterification methods and use of phosphorus pentoxide[J]. journal of dentistry, 2008, 36(3): 171-177.
- Related articles
- Related Qustion
YK-11 is a synthetic steroidal selective androgen receptor modulator (SARM).It is a gene-selective partial agonist of the androgen receptor (AR) and does not induce the physical interaction between the NTD/AF1 and LBD/AF2 (known as the N/C....
Aug 16,2022APITedizolid is the first FDA-approved second-generation oxazolidinone antibiotic, which is clinically used to treat skin and skin tissue infections, and its efficacy is non-inferior to linezolid.....
Aug 17,2022APIPhosphorus pentoxide
1314-56-3You may like
Phosphorus pentoxide manufacturers
- Phosphorus pentoxide
-

- $10.00 / 25kg
- 2024-11-07
- CAS:1314-56-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 200000
- Phosphorus pentoxide
-

- $0.00 / 1kg
- 2024-10-25
- CAS:1314-56-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100tons
- Phosphorus pentoxide
-

- $4340.00 / 1tons
- 2024-10-24
- CAS:1314-56-3
- Min. Order: 1tons
- Purity: 99%
- Supply Ability: 100tons