The introduction of Trastuzumab Deruxtecan
Feb 4,2024
Description
Trastuzumab deruxtecan, a HER2-directed antibody and DNA topoisomerase I inhibitor conjugate, is being developed for the treatment of HER2-expressing solid tumors, including breast cancer, gastric cancer, colorectal cancer and non-small cell lung cancer by Daiichi Sankyo Company Ltd in collaboration with AstraZeneca[1]. Based primarily on the results of the phase 2 DESTINY-Breast01 trial, trastuzumab deruxtecan was recently approved in the USA under accelerated approval for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting.
Structure
The drug, which incorporates the same antibody as trastuzumab emtansine (Kadcyla), contains a HER2- directed antibody, trastuzumab, conjugated to topisomerase I inhibitor DXd via an enzymatically cleavable maleimide glycine-glycine-phenylalanine-glycine (GGFG) peptidic linker. The linker−DXd intermediate, known as deruxtecan, is conjugated to trastuzumab via interchain cysteine residues present on the antibody, with an average of eight deruxtecan units attached to each trastuzumab antibody (DAR ≈ 8).

Biological activity
The observed therapeutic effect of trastuzumab deruxtecan relies on the overexpression of HER2 in many cancer types, facilitating antibody-driven binding of the ADC with HER2 receptors in cancer cells. After binding the ADC to the surface of the targeted cell, internalization and subsequent protease-mediated cleavage of the linker portion of deruxtecan take place, releasing DXd as the active species. A camptothecin derivative of the known topisomerase I inhibitor exatecan, DXd inhibits DNA replication, tumor cell apoptosis, and cell cycle arrest by binding with and inhibiting topoisomerase I-DNA complexes[2]. Interestingly, the linker technology used in trastuzumab deruxtecan varies from that in many other ADCs currently approved for human use, enabling a significantly higher DAR and the maximum theoretical DAR possible for conventional interchain cysteine conjugation. The increased drug loading enables a substantially higher delivery of payload to tumor cells, even cells with low HER2 expression. This high DAR is possible because of a favorable stability and clearance profile, which is often a liability with other ADC types. In clinical trials of 184 patients treated with the drug, 60% of patients dosed with trastuzumab deruxtecan observed tumor shrinkage, leading to a median response duration of 14.8 months.
References
[1] Keam, Susan J. “Trastuzumab Deruxtecan: First Approval.” Drugs 80 5 (2020): 501–508.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
- Related articles
- Related Qustion
- How to synthesize Trastuzumab Deruxtecan? Feb 4, 2024
Preparation of the final drug product, trastuzumab deruxtecan, necessitated conjugation of deruxtecan to the anti-HER2 monoclonal antibody trastuzumab, generating the ADC with a loading of approximately eight deruxtecan units per antibody.
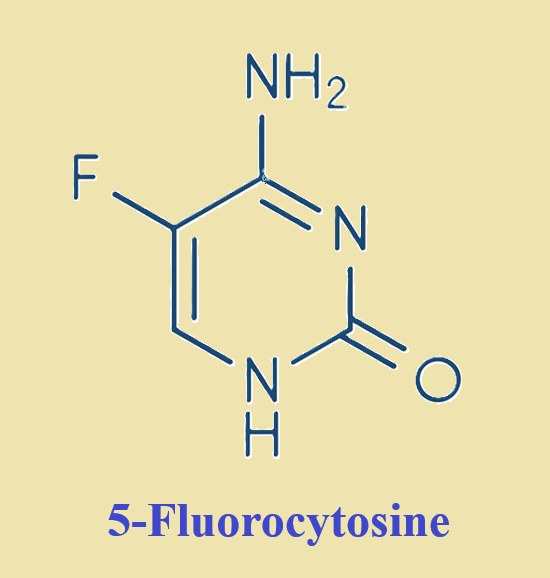
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Nov 1,2024Biochemical EngineeringPreparation of the final drug product, trastuzumab deruxtecan, necessitated conjugation of deruxtecan to the anti-HER2 monoclonal antibody trastuzumab, generating the ADC with a loading of approximately eight deruxtecan units per antibody.....
Feb 4,2024APITrastuzumab Deruxtecan
1826843-81-5You may like
Trastuzumab Deruxtecan manufacturers
- Trastuzumab deruxtecan
-

- $728.00 / 1mg
- 2024-11-15
- CAS:1826843-81-5
- Min. Order:
- Purity: SEC-HPLC:98.49%
- Supply Ability: 10g