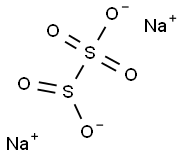
The Multifaceted Role of Sodium Metabisulfite in Modern Chemistry
May 29,2024
Introduction
Sodium metabisulfite, a chemical compound with the formula Na2S2O5, serves as a versatile agent widely utilized in various industrial and chemical processes. This inorganic salt, also known as sodium pyrosulfite, is recognized for its potent reducing properties and its ability to act as a preservative, antioxidant, and disinfectant. Its effectiveness in preventing oxidative decay makes it particularly valuable in sectors like food preservation, winemaking, and even in environmental management where it helps in detoxifying industrial effluents. The compound's diverse functionalities not only underscore its chemical significance but also highlight its role in enhancing product longevity and safety across multiple industries.

Figure 1 Characteristics of Sodium metabisulfite
Synthesis of Sodium Metabisulfite
The commercial production of sodium metabisulfite typically involves the reaction of sodium hydroxide (NaOH) with sulfur dioxide (SO2), a process that forms sodium bisulfite (NaHSO3), which is then further treated to yield sodium metabisulfite. This reaction is efficient and cost-effective, allowing for large-scale production. The purity of the reagents and the control of reaction conditions are crucial to achieving high-quality sodium metabisulfite with minimal impurities.
Main Components
Sodium metabisulfite is primarily composed of sodium (Na+), sulfur (S), and oxygen (O) atoms in a ratio that forms a stable, crystalline powder. This specific arrangement of atoms gives sodium metabisulfite its chemical stability and reactive capabilities. The presence of the sulfite ion (SO3^2-) in sodium metabisulfite is particularly significant, as it is responsible for many of its reducing and preservative actions. Furthermore, this ion plays a critical role in its antioxidant properties, making it highly effective in preventing oxidative damage in various organic materials and in scavenging free radicals in different environments.
Applications
Sodium metabisulfite finds extensive use across a variety of industries due to its chemical properties. In the food industry, it is used as a preservative and antioxidant, helping to prevent oxidation and maintain the freshness and color of the product. It also acts as a disinfectant in winemaking, where it is used to inhibit the growth of bacteria and fungi and to preserve the natural color of wines.
In the field of water treatment, sodium metabisulfite is employed to remove excess chlorine from drinking water. Additionally, it serves as a key component in the photographic industry for developing and fixing photographs. The compound is also used in the textile and pulp industries as a bleaching agent. Beyond these applications, sodium metabisulfite is instrumental in the mining industry for ore processing, aiding in the separation of minerals and metals from their ores. It also acts as a source of sulfur dioxide in organic synthesis, serving as a reagent in various chemical reactions. Furthermore, its use as a reducing agent helps in the treatment and conditioning of waste before disposal, underscoring its environmental benefits.
Storage Methods
Proper storage of sodium metabisulfite is crucial to maintain its effectiveness and ensure safety. It should be stored in a cool, dry place away from moisture and heat. Exposure to moisture can lead to the release of sulfur dioxide gas, which is toxic and can cause respiratory problems. Containers used to store sodium metabisulfite should be tightly sealed to prevent the ingress of moisture. Additionally, it should be kept away from oxidizing agents, as mixing these substances can lead to hazardous reactions.
Conclusion
Sodium metabisulfite is an indispensable chemical compound with wide-ranging applications in various industries. Its ability to act as a preservative, antioxidant, and disinfectant make it a valuable resource in the chemical toolkit. Understanding its synthesis, components, applications, and proper storage methods is essential for professionals in the chemistry field to utilize this compound safely and effectively. As the demand for versatile and efficient chemical agents continues to grow, sodium metabisulfite is likely to remain a key player in the chemical industry for years to come.
References
[1]Madan, Vishal, Stephen L. Walker, and Michael H. Beck. "Sodium metabisulfite allergy is common but is it relevant?."Contact dermatitis57.3 (2007): 173-176.
[2]García‐Gavín, Juan, Joana Parente, and An Goossens. "Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. A case series and literature review."Contact dermatitis67.5 (2012): 260-269.
- Related articles
- Related Qustion
- Sodium Metabisulfite: Uses, Synthesis and Hazards Nov 24, 2022
The passage introduces the uses, synthesis and hazards of sodium metabisulfite, also mention the reaction with water of it.
- a challenging allergen:sodium metabisulfite Apr 11, 2022
Sodium metabisulfite appears as a white crystalline or powder solid with a slight sulfur odor. Toxic by inhalation. Sodium disulfite is an inorganic sodium salt composed. of sodium and disulfite ions
N-Iodosuccinimide (NIS) is a pivotal reagent in the realm of synthetic chemistry, renowned for its versatility and efficiency.....
May 29,2024APIHuperzine A, a compound derived from the Chinese club moss Huperzia serrata, its significant impact on neuroprotection and cognition enhancement.....
May 29,2024APISodium metabisulfite
7681-57-4You may like
Sodium metabisulfite manufacturers
- Sodium metabisulfite
-

- $230.00 / 1ton
- 2024-07-15
- CAS:7681-57-4
- Min. Order: 5ton
- Purity: 97%
- Supply Ability: 3000tons
- Sodium metabisulfite
-

- $377.00 / 1ten
- 2024-07-15
- CAS:7681-57-4
- Min. Order: 1ten
- Purity: 99%
- Supply Ability: 1000ton
- Sodium metabisulfite
-

- $6.00 / 1kg
- 2024-07-10
- CAS:7681-57-4
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month