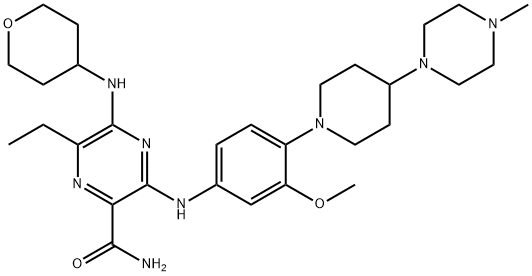
The Pharmacodynamics and Synthetic method of Gilteritinib
Dec 28,2023
Description
Gilteritinib, an oral inhibitor of FMS‐like tyrosine kinase 3 (FLT3), is a standard treatment for FLT3‐mutated acute myeloid leukemia[1]. It is co-developed by Astellas and Kotobuki Pharmaceuticals.
This drug is approved in Japan for the treatment of anaplastic lymphoma kinase (ALK) and relapsed or refractory acute myeloid leukemia (AML) with FLT3 mutation. Gilteritinib also binds to and inhibits the wildtype and mutant forms of ALK, preventing cell proliferation in cancer cell types that overexpress the mutation. In November 2018, the USFDA approved the drug for the treatment of adults with relapsed FLT3 positive refractory AML as detected by a USFDA-approved test.
Pharmacodynamics
Gilteritinib, a pyrazine carboxamide derivative, is a selective inhibitor of multiple receptor tyrosine kinases, including FLT3 and AXL (geometric mean 50% inhibitory concentration 0.29 and 0.73 nmol/L, respectively). In vitro, gilteritinib inhibited FLT3 receptor signaling and cell proliferation in cell lines expressing FLT3. Gilteritinib (a type I inhibitor) demonstrated potent activity against FLT3 with ITD and TKD mutations, including point mutations known to confer resistance to type II inhibitors and, to a lesser degree, against the F691 gatekeeper mutation. Computational modeling indicated that gilteritinib binds to FLT3 at the ATP binding site, far from the D835 position in the activation loop; it hydrophobically interacts with FLT3 at the 691 positions[2].
In vitro and animal studies showed that gilteritinib inhibits the phosphorylation of FLT3 and its downstream targets. In vivo gilteritinib distributed at high levels in tumors and was associated with durable inhibition of phosphorylated FLT3 and STAT5, resulting in tumor regression (xenograft model) and decreased leukemic burden
and prolonged survival (intra-bone transplantation model of FLT3 driven AML). In a first-in-human, open-label, phase 1/2 study in patients with relapsed or refractory AML (NCT02014558), treatment with gilteritinib 120 mg/day was associated with substantial (>90%), rapid (within 24 h) and sustained FLT3 phosphorylation inhibition, as assessed by a plasma inhibitory activity assay.
Synthetic method
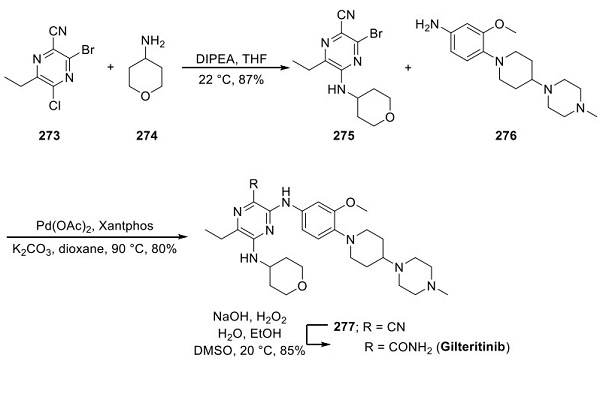
Although the synthesis of gilteritinib has been reported by Astellas, no yields were reported throughout the synthetic sequence. A separate approach has been reported in a Chinese patent in greater detail[3]. Chloropyrazine 273 was treated with aminopyran 274 in the presence of a base. The resulting aminopyrazine 275 underwent a palladium-catalyzed amination reaction with aniline 276, a subunit present in Ariad’s ALK/EGFR dual inhibitor brigatinib, approved in 2017. The resulting nitrile 277 underwent hydrolysis to furnish gilteritinib in 85% yield.
References
[1] Takeo Yasu. “Determination of the concentration of gilteritinib in human plasma using HPLC.” Biomedical Chromatography 35 4 (2021): e5028.
[2] Dhillon, Sohita. “Gilteritinib: First Global Approval.” Drugs 79 3 (2019): 331–339.
[3] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGlasdegib was approved in 2018 for the treatment of newly diagnosed acute myeloid leukemia (AML) patients at least 75 years of age who are not appropriate candidates for intensive induction chemotherapy.....
Dec 28,2023APIGilteritinib
1254053-43-4You may like
- Gilteritinib
-

- $34.00 / 1mg
- 2025-12-16
- CAS:1254053-43-4
- Min. Order:
- Purity: 99.32%
- Supply Ability: 10g
- Gilteritinib
-
- $0.00 / 10mg
- 2025-12-16
- CAS:1254053-43-4
- Min. Order: 10mg
- Purity: 95%+
- Supply Ability: 10000000
- Gilteritinib
-

- $0.00 / 1g
- 2025-09-11
- CAS:1254053-43-4
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month