The Pharmacology of ketoprofen
Apr 8,2022
Introduction
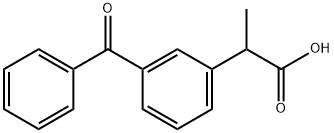
Ketoprofen (3-benzoyl-α-methylphenylacetic acid) is a 2-arylpropionic acid potent non-steroidal anti-inflammatory drug. It was first synthesized by French chemist Rhone Poulenc in 1967. In 1973, it was introduced into France and the United States as an anti-inflammatory drug [1]. It has good effects on rheumatism, rheumatoid arthritis, myelitis and gout, and its anti-inflammatory effect is stronger than that of ibuprofen. Ibuprofen. At the same dose, its anti-inflammatory and analgesic effect is 150 times that of aspirin, its antipyretic effect is 4 times that of indomethacin and 100 times that of aspirin. Because ketoprofen has high efficacy, short half-life, It has the advantages of simple metabolism and few and mild adverse reactions, and has been widely used in the treatment of various types of pain, inflammatory symptoms, colds and post-operative anti-inflammatory analgesia.
Properties of ketoprofen
Ketoprofen is a chemical that comes in the form of a white crystalline powder; odorless or nearly odorless[2]. It is very soluble in methanol, soluble in ethanol, acetone or ether, and almost insoluble in water. The melting point is about 93-96 °C. For the arylalkanoic acid compounds. Has analgesic, anti-inflammatory and antipyretic effects. The anti-inflammatory effect is stronger than that of ibuprofen, with less side effects and low toxicity. Oral and easily absorbed from the gastrointestinal tract. After 1 administration, the peak plasma concentration can be reached in about 0.5 to 2 hours. t 1/2 is 1.6 to 1.9 hours. In the blood and plasma protein binding force is extremely strong. The excretion rate from urine is 30% to 90% within 24 hours. Mainly excreted in the form of glucuronic acid conjugates. For rheumatoid arthritis, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis and gout, etc.

Picture 1 Ketoprofen powders
Pharmacology
Ketoprofen is a non-steroidal anti-inflammatory, analgesic and antipyretic drug, which is characterized by high efficiency and low toxicity[3], and its curative effect is better than that of the same drug - ibuprofen. Oral ketoprofen is rapidly absorbed and eliminated, and ordinary tablets are generally administered 3-4 times a day. Therefore, it is of great significance to make ketoprofen into a controlled-release preparation for reducing the fluctuation of blood concentration and improving the bioavailability of the drug[4-5]. Osmotic pump preparation is a typical representative of controlled-release preparations. It has the characteristics of zero-order drug release, constant drug release rate, and less influence by the drug release environment. It can avoid the phenomenon of large fluctuations in blood drug concentration caused by ordinary oral preparations. Greatly improve drug safety and effectiveness.
Market development and prospect of ketoprofen
S-ketoprofen is the active enantiomer of (RS)-ketoprofen, developed by Italian Menarini Company and first listed in Spain in 1996. Its anti-inflammatory and analgesic effects are two of the racemates. In clinical practice, only half the dosage of the racemate can achieve the same therapeutic effect. At present, there is no domestic manufacturer of this drug, so it is necessary to research and develop S-ketoprofen. The methods for obtaining S-ketoprofen mainly include asymmetric synthesis, enzymatic resolution, and diastereomeric crystallization resolution. Among them, asymmetric synthesis and enzymatic resolution are not yet available in our country at the present stage, and the crystallization and resolution of diastereomers with chiral amines has the advantages of simple operation and stability.
Ketoprofen interactions with other drugs
Concomitant use of alcohol or other NSAIDs after taking ketoprofen can increase gastrointestinal side effects and may cause ulcers. When ketoprofen is used together with aspirin or other salicylic acid drugs, the efficacy cannot be increased, but the incidence of gastrointestinal side effects and bleeding tendency increases. Concomitant use of ketoprofen with anticoagulants increases the risk of bleeding. Ketoprofen can enhance the effect of antidiabetic drugs and reduce the antihypertensive effect of antihypertensive drugs; ketoprofen and corticosteroids can be used together, which can significantly reduce the symptoms of inflammation. Ketoprofen should not be used with methotrexate to prevent poisoning. When ketoprofen is used with probenecid, verapamil, and nifedipine, the dose should be reduced; when ketoprofen is used with digoxin, the dose of digoxin should be adjusted.
Reference
1 Kantor T G. Ketoprofen: a review of its pharmacologic and clinical properties[J]. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 1986, 6(3): 93-102.
2 Liversidge G G. Ketoprofen[M]//Analytical profiles of drug substances. Academic Press, 1981, 10: 443-471.
3 Mazieres B. Topical ketoprofen patch[J]. Drugs in R & D, 2005, 6(6): 337-344.
- Related articles
- Related Qustion
- Ketoprofen - A Journey Through Its Wonders and Applications Oct 8, 2024
This comprehensive review delves into the chemistry, pharmacology, clinical applications, and emerging research surrounding Ketoprofen.
- Ketoprofen: Synthesis, Side effects, and Drug interactions Jul 11, 2024
Ketoprofen, chemistry 3-benzoyl by name-Alpha-Methyl toluylic acid, is a non-steroidal anti-inflammatory analgesics, has anti-inflammatory, analgesic, analgesic effects.
Retinoic acid, the molecular formula is C20H28O2, is the metabolic intermediate product of vitamin A in the body.....
Apr 8,2022APITorcitabine (b-L-2u-deoxycytidine) (L-dC) is the b-L-enantiomer of the natural nucleoside D-cytidine. The drug is currently under development by Idenix as an antiviral agent for the treatment of chronic hepatitis B virus infection. Torcitab....
Apr 8,2022APIKetoprofen
22071-15-4You may like
- Ketoprofen
-

- $0.00 / 1g
- 2024-11-06
- CAS:22071-15-4
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Ketoprofen
-

- $45.00 / 500mg
- 2024-11-05
- CAS:22071-15-4
- Min. Order:
- Purity: 99.67%
- Supply Ability: 10g
- Ketoprofen
-

- $45.00 / 500mg
- 2024-11-05
- CAS:22071-15-4
- Min. Order:
- Purity: 99.67%
- Supply Ability: 10g