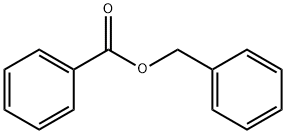
The preparation method of benzyl benzoate
Aug 12,2022
Introduction
Benzyl benzoate (BB) is one of the oldest drugs used for the treatment of scabies and is recommended as the “first-line intervention” for the cost-effective treatment of the disease.
Since benzyl benzoate is a pyrolytic product from benzaldehyde at 350°, it was of interest to study the behavior of benzyl benzoate under similar conditions. The results show that about half of the ester is recoverable after two hours of heating. The products formed are best explained by assuming an initial disproportionation into benzoic anhydride and dibenzyl ether, the former appearing as such and the latter decomposing6 further into toluene and benzaldehyde. The reaction mixture was carefully searched for stilbene, phenanthrene, benzene, diphenyl and dibenzyl ether but none of these substances could be identified.
Properties
At 350° benzyl benzoate undergoes a reaction of disproportionation. After two hours’ heating, the major products of change are benzoic an[1]hydride, toluene and benzaldehyde. Smaller amounts of benzoic acid were also indentified. Negative searches were made for stilbene, phenanthrene, benzene, diphenyl and dibenzyl ether in the products of reaction[1].
Investigations have been in progress for the preparation of benzyl benzoate, a miticide, by means other than its standard preparation from compounds derived from toluene. The most promising process has been the conversion of benzene to benzyl chloride by chloromethylation (Equation 1) and the dry esterification of the latter with sodium benzoate in the presence of tri[1]ethylamine (Equation 2)
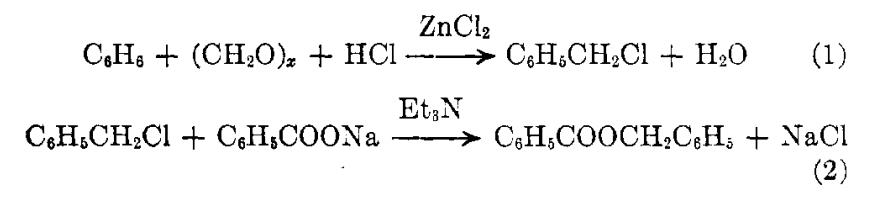
Picture 1 The conversion of benzene to benzyl chloride
The first step in this process has been reported. Rueggeberg, Ginsburg, and Frantz have reported some laboratory results on the dry esterification step. It is the purpose of this report to give additional laboratory data and also pilot plant data for this reaction. Considerable work has been done on the preparation of benzyl esters under anhydrous conditions. The methods have been reviewed by Gomberg and Buchler. For the most part long periods of heating at high temperatures were required, and in many cases low yields of the esters were obtained. Preparation of the benzyl esters in the presence of organic bases is first described in a patent assigned to the Badische Anilin- & Soda Fabrik, and Volwiler and Vliet have prepared certain benzyl esters using diethylamine as a catalyst.
Benzyl benzoate from benzyl chloride and sodium benzoate.
As a result of wartime restrictions on the availability of toluene and, hence, belizaldehyde, production processes for the manufacture of the miticide benzyl benzoate other than by the Claisen condensation of benzaldehyde had to be investigated[2]. Simultaneously, improved processes for production of benzyl chloride by chloromethylation of benzene and of benzoic acid through the aluminum-chloride-catalyzed reaction between benzene and phosgene were also studied in this laboratory. These developments led to two methods for carrying out the double decomposition between benzyl chloride and sodium benzoate to form benzyl benzoate. One of them depends upon the presence of water as reaction solvent for sodium benzoate, as first described by Gomberg and Buchler; the other relies on the catalytic activity of a small amount of tertiary amine in the absence of any reaction solvent other than that supplied by the reacting materials.
The amine-catalyzed reaction on a slurry of dry sodium benzoate in benzyl chloride is interesting. Despite the fact that practically quantitative yields of aromatic acid esters may be obtained easily, this method has strangely remained out of the chemical literature since its first appearance as a German patent in 1912. Scelba reports that dry sodium benzoate, heated with a slight excess of benzyl chloride at 170-175 ° C. for 24 hours, produces. a 70-75% yield of benzyl benzoate. According to the German patent, a small quantity of triethylamine lowers the reaction temperature required for this same reaction to about 130- 140° C., the reaction time to about one hour, and raises the ester yield to 95% or higher.
Reference
I. D. Tharp, H. A. Nottorf, C. H. Herr, T. B. Hoover, R. B. Wagner, C. A. Weisgerber, J. P. Wilkins, and F. C. Whitmore. Industrial & Engineering Chemistry 1947, 39, 10, 1300-1302
Walter H. C. Rueggeberg, Abram Ginsbu'rg, and Russell K. Frantz. Benzyl Benzoate from Benzyl Chloride and Sodium Benzoate. Industrial & Engineering Chemistry 1946, 38, 2, 207-211.
- Related articles
- Related Qustion
- Benzyl Benzoate: An Overview of its Synthesis, Composition, Applications, and Storage May 23, 2024
Benzyl benzoate, a chemical compound with significant applications across various industries, has been widely studied for its versatility and efficacy.
- Side effects of Benzyl benzoate Nov 23, 2021
Benzyl benzoate occurs naturally in Peruvian and Tulu balsams as well as other essential oils. It was used in Vietnam War as a repellant for ticks and mites. Benzyl benzoate was used as an antispasmodic, but has been replaced with more effe
Benzyl benzoate
120-51-4You may like
- Benzyl benzoate
-

- $1.00 / 1g
- 2025-04-15
- CAS:120-51-4
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- Benzyl benzoate
-

- $3.50 / 1kg
- 2025-04-15
- CAS:120-51-4
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 3000tons/month
- Benzyl benzoate
-

- $5.00 / 25kg
- 2025-04-15
- CAS:120-51-4
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 200mt/year