Triphosgene: History and Advantage
Aug 9,2024
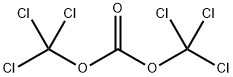
Triphosgene, or bis(trichloromethyl) carbonate or BTC, is a convenient substitute for the extremely toxic phosgene gas. It is a stable crystalline solid with a melting point of 80 °C, but it decomposes at temperatures above 200 °C.

History
The first preparation of triphosgene was reported by Councler in 1880, in which its synthesis was accomplished through liquid-phase photochlorination of dimethyl carbonate. The physical and chemical properties of triphosgene were documented by Hentschel in 1887. Its solid-state X-ray characterization data was then reported by Sorensen in 1971. Interestingly, the utility of triphosgene in synthetic reactions remained relatively unexplored until it was “rediscovered” in the late 1980s. Since then, triphosgene has proven to be a convenient replacement for phosgene. It has been widely used in many laboratory and industrial reactions. Because of its stable crystalline form, triphosgene provides safety benefits that consequently enable its transportation, storage, and handling and are more practicable than phosgene gas. Furthermore, triphosgene offers operational simplicity for typical laboratory-scale reactions since the exact amounts of this reagent can be measured under standard safety protocols[1].
Advantage
The main reason for such intensive application of BTC in synthesis may be ascribed to two different aspects, namely: (i) the use of triphosgene as a safer solid and easier to handle compared to phosgene and (ii) the specific reactivity of triphosgene that enables the preparation of unsymmetrical ureas, carbamoyl chlorides and isocyanates, unsymmetrical carbonates, and carbamates.
How is triphosgene converted to phosgene?
Triphosgene was decomposed quantitatively to phosgene by chloride ions. The chloride ion, under these reaction conditions, is a strong nucleophile that attacks the carbonyl carbon of triphosgene, giving diphosgene and phosgene. In turn, diphosgene reacts with chloride ions to give two molecules of phosgene. The chloride ion is needed in catalytic amounts since it is not consumed in the overall process. The reaction course was monitored by IR spectroscopy (React-IR), showing that diphosgene was an intermediate. The methanolysis of triphosgene in deuterated chloroform, monitored by proton NMR spectroscopy, gave methyl chloroformate and methyl 1,1,1-trichloromethyl carbonate in about a 1:1 ratio as primary products[2].
References
[1] Lucia Pasquato. “Conversion of Bis(trichloromethyl) Carbonate to Phosgene and Reactivity of Triphosgene, Diphosgene, and Phosgene with Methanol1.” The Journal of Organic Chemistry 65 24 (2000): 8224–8228.
[2] Moshood O. Ganiu . “A decade review of triphosgene and its applications in organic reactions.” Tetrahedron 76 47 (2020): Article 131553.
- Related articles
- Related Qustion
- Introduction of Triphosgene Jan 25, 2022
Triphosgene is also known as solid phosgene. Its chemical name is bis (Trichloromethyl) carbonate, and its English name is bisgriehloromethyl) carbonate or triphosgene, abbreviated as BTC. Triphosgene
Ethanol, also known as ethyl alcohol or alcohol, is a volatile, flammable, colourless liquid with the molecular formula C2H5OH.....
Aug 9,2024Organic reagentsCumene, also known as isopropylbenzene or 1-methylbenzene, is a flammable, volatile, colourless liquid with an odour similar to petrol.....
Aug 9,2024APITriphosgene
32315-10-9You may like
- Triphosgene
-

- $0.00 / 25KG
- 2024-09-13
- CAS:32315-10-9
- Min. Order: 1KG
- Purity: ≥99.5%
- Supply Ability: 2000mt/year
- Triphosgene
-

- $0.00 / 25KG/Tin
- 2024-09-13
- CAS:32315-10-9
- Min. Order: 1000Kg/Drum
- Purity: 99.5
- Supply Ability: 10000
- Triphosgene
-

- $1.70 / 1KG
- 2024-08-21
- CAS:32315-10-9
- Min. Order: 10KG
- Purity: 99%
- Supply Ability: 10000kg