Vanadium monocarbide:Structure,Uses,Preparation
Apr 11,2024
Vanadium monocarbide is a tough and refractory ceramic material.
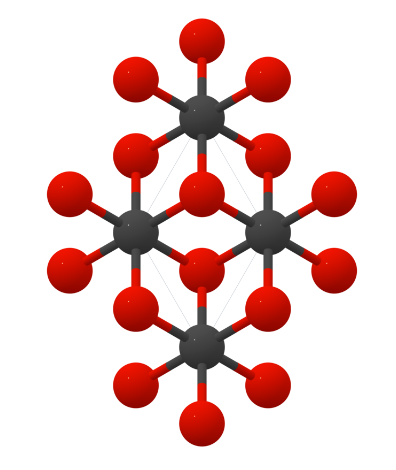
Crystal structure
Vanadium monocarbide has a hexagonal structure.
Uses
Vanadium monocarbide is a thermodynamic material used as an activation energy source to produce nitrides and carbides. Vanadium has a high melting point, which makes it ideal for applications where high temperatures are involved.
Preparation
To produce vanadium monocarbide, the metal must be heated to a temperature of approximately 2200 degrees Celsius. This material has been shown to have a particle size of less than 1 micrometer and can be activated by light or heat. The elemental composition of vanadium monocarbide is approximately 50% V and 50% C, with some impurities such as nitrogen atoms present.
Related compounds
Vanadium forms with carbon plenty of chemical compounds: vanadium monocarbide VC1–x having the broad homogeneity range with the highest C/V ratio being extremely far from the stoichiometry, several modifications of vanadium semicarbide:
α-V2+xC (Pbcn), β-V2±xC (P63/mmc) and β′-V2+xC (P(–3)m1, P(–3)1m, P312, Pnma, R(–3)m, P4/mmm, I41/amd, P3m1), some ordered and metastable phases: V5C3±x (I4/mcm), V3C2±x (R(–3)m, Immm, C2221, I4/mmm, C2/m, Immm, P(–3)m1), ζ-V4C3–x (or ξ-V3C2+x; R(–3)m, Pnma, P(–3)m1, C2/c, Pm(–3)m), V5C4±x (P(–1), C2/m, I4/m), V6C5±x (P3112, P31, P32, C2/m, C2/c, C2), V7C6±x (R(–3)) and V8C7±x (P4132, P4332), some subcarbides VxC (or more probably ordered V-C solid solutions) and metallocarbohedrene V8C12.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTungsten silicides appear suitable for operation in extreme conditions. This article will introduce the crystal structure and properties of tungsten based silicides.....
Apr 11,2024Inorganic chemistryVanadium monocarbide
12012-17-8You may like