Vanadyl Acetylacetonate: A Versatile Coordination Compound in Chemistry
Oct 22,2024
Introduction
Vanadyl acetylacetonate (VO(acac)₂), also known as vanadium(IV) acetylacetonate, is a well-known coordination compound with the formula VO(C₅H₇O₂)₂. It is widely recognized in the chemical community for its unique properties and versatile applications. This compound is typically used in catalysis and material science and as a precursor for various vanadium-containing materials.

Figure 1 Characteristics of Vanadyl acetylacetonate
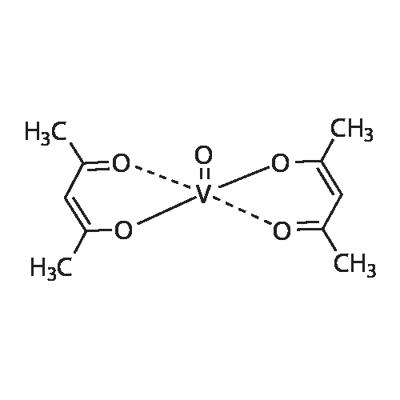
Structure
Vanadyl acetylacetonate, VO(acac)2, is the simplest β-diketonate. Its structure was studied by Fedorova et al. using X-ray diffraction and Fourier transform IR spectroscopy. The structure of the VO(acac)2 molecule is shown in the figure below. The length of the V(1)–O(1) double bond is 0.16 nm, and this bond is 0.03 nm shorter than the four equatorial V–O bonds. The lengths of these bonds, V(1)–O(21), V(1)–O(12), and V(1)–O(22), are 0.196–0.197 nm. The sixth ligand, for example, oxygen, in the VO4 pyramid may be coordinated to the trans position relative to the V=O group, with or without changes in the double bond.

To block the free bond and prevent V(IV) → V(V) oxidation, use is made of allylic alcohols, which form π-complexes with VO(acac)2.
Hydrolysis
Vanadyl acetylacetonate dissolves in chloroform, methanol, ethanol, acetone, benzene, and nitrobenzene to form adducts, but is insoluble in water. Given that, to produce vanadium dioxide films, it is heated in the presence of water, hydrolysis processes are possible. There is spectrophotometric evidence that, in an aqueous methanol solution, Vanadyl acetylacetonate is oxidized by atmospheric oxygen to form vanadium(V) hydroxomethoxooxo(pentane-2,4-dionate), with detachment of one of the chelate ligands in the form of acetylacetone.
In acidified aqueous solutions, VO(acac)2 experiences two-step hydrolysis. In this process, the hydrolysis rate of the second acetylacetonate ring is 150 times lower than that of the first ring.
Preparation
Vanadyl acetylacetonate was prepared by reacting vanadyl sulfate and acetylacetone. X-ray diffraction characterization of the as prepared VO(acac)2 (DRON 4-07 diffractometer, Cu Kα radiation, λ = 1.54178 Å, angular range 2θ = 10° to 65°, step size of 0.2°) showed that the synthesized vanadyl acetylacetonate was phasepure.1
References:
[1] R. N. NENASHEV. Thermal decomposition of vanadyl acetylacetonate[J]. Inorganic Materials, 2015, 51 9. DOI:10.1134/S0020168515090150.
- Related articles
- Related Qustion
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIMethyl anthranilate (MA) is the characteristic flavor compound of Concord grapes and also appears in several essential oils such as neroli and bergamot oils.....
Oct 24,2024APIVanadyl acetylacetonate
3153-26-2You may like
Vanadyl acetylacetonate manufacturers
- Vanadyl Acetylacetonate
-

- $150.00 / 1kg
- 2025-04-16
- CAS:3153-26-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500kg
- Vanadyl acetylacetonate
-

- $0.00 / 1KG
- 2025-04-15
- CAS:3153-26-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Vanadyl acetylacetonate
-

- $0.00 / 25KG
- 2025-03-21
- CAS:3153-26-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month