What is Poly(Acrylic Acid)(PAA)?
Mar 12,2021
Definition
Poly(acrylic acid) (PAA) is a polymer of acrylic acid, which has a carboxylic group on each monomer unit. This polymer becomes a polyelectrolyte in water via dissociation of the acid groups.Poly(acrylic acid) is a synthetic high-molecular weight polymer of acrylic acid. The IUPAC name is poly(1-carboxyethylene). They may be homopolymers of acrylic acid, or crosslinked with an allyl ether of pentaerythritol, allyl ether of sucrose, or allyl ether of propylene. In a water solution at neutral pH, PAA is an anionic polymer, i.e. many of the side chains of PAA will lose their protons and acquire a negative charge. This makes PAAs polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume.
Commodity Products
Dry PAAs are sold as white, fluffy powders that are frequently used as gels in cosmetic and personal care products. Their role in cosmetics is to suspend solid in liquids, prevent emulsions from separating and control the consistency in flow of cosmetics. Carbomer codes (910, 934, 940, 941, and 934P) are an indication of molecular weight and the specific components of the polymer. For many applications PAAs are used in form of alkali metal or ammonium salts, e.g. sodium polyacrylate. In the dry powder form, the positively charged sodium ions are bound to the polyacrylate, however in aqueous solutions the sodium ions can dissociate. Instead of an organized polymer chain, this leads to a swollen gel that can absorb a high amount of water.

Chemical Structure and Synthesis
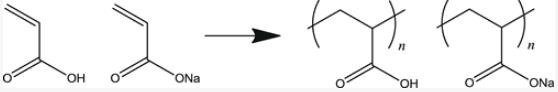
Poly(acrylic acid) (PAA) has a carboxyl group on every two carbon atoms of the main chain. It has high negative charge density when all carboxyl groups dissociate. This polymer and poly(sodium acrylate) (NaPAA) are thus one of the most abundantly used water-soluble anionic polyelectrolytes, e.g., dispersing agent, superabsorbent polymer, ion-exchange resin, etc. Furthermore, due to low toxicity, they are used as a food additive. They are synthesized industrially by radical polymerization of acrylic acid or sodium acrylic acid as shown in Fig. 1. Therefore, the molar mass distribution of the resultant polymer is broad.
Application
Acrylic acid has traditionally been used as the raw material for acrylic esters – methyl acrylate, ethyl acrylate, butyl acrylate and 2-ethylhexyl acrylate. These bulk acrylates were originally used to produce solvent-based acrylic resins but environmental concerns over solvent use led to the development of water-based acrylics. Applications for water-based acrylics are primarily in decorative, masonry and industrial coatings but other uses include adhesives, paper and leather coatings, polishes, carpet backing compounds and tablet coatings.
Another major use for acrylic acid is the manufacture of polyacrylates which are used as thickeners, dispersants and rheology controllers. Acrylic acid is also employed as a comonomer with acrylamide in anionic polyacrylamide and to produce hydroxyacrylates for use in industrial coating formulations.
From the mid-1980s, two new applications - superabsorbent polymers (SAPs) and detergent polymers - have emerged. SAPs are cross-linked polyacrylates with the ability to absorb and retain more than 100 times their own weight in liquid. They have experienced very strong growth, primarily in baby diapers (nappies), although the US and West European markets have now matured. SAPs account for over 30% of world acrylic acid consumption.
Polymer synthesis
Free radical polymerization is still the most common industrial for production of polymers. For the free radical polymerization of acrylic acid, most commonly thermochemical initiators such as potassium persulfate and AIBN are used. The polymerization can take several hours to complete but can be accelerated by increasing the temperature and pressure[clarification needed]. PAA is widely used in dispersants and since the molecular weight has a significant impact on the rheological properties and dispersion capacity, it is crucial to have control over the molecular weight distribution. RAFT polymerization was used to obtain PAA with a defined molecular weight, leading to improved dispersion properties. This was only possible in low AA/CTA ratios, at higher ratios transfer to the solvent was observed that had a negative effect on control over the reaction. Block copolymers of PAA have been prepared that are responsive to pH and temperature stimuli, demonstrating the possibility to be used for drug delivery purposes. ATRP has been used to grow PAA brushes from the surface. One reported approach was with surface-initiated ATRP of tert-butyl acrylate that was subsequently deprotected through pyrolysis to form the carboxylic acid. These PAA brushes can form chelate complexes with silver ions that exhibit antibacterial activity.
- Related articles
- Related Qustion
- Carbomer: A Critical Component in Modern Chemistry May 29, 2024
In the expansive field of polymer chemistry, carbomer stands out as a versatile and vital synthetic compound.
- POLY(ACRYLIC ACID): Application, Synthesis and Hazard Apr 13, 2023
Poly(acrylic acid) is a polymer of acrylic acid, which has a carboxylic group on each monomer unit. It is often used as gels in cosmetics and personal care products.
- Applications and Side effects of Carbomer Apr 14, 2022
Carbomer is a term used for a series of polymers primarily made from acrylic acid. Carbomers are white, fluffy powders but are frequently used as gels in cosmetics and personal care products.
Hydroxychloroquine is a 4-aminoquinolone that is used to prevent and treat malaria, caused by parasitic protozoan from the Plasmodium genus.....
Mar 9,2021Antimicrobial agentTetrabutylammonium fluoride hydrate is known to be a source of F? anion, used in nucleophilic fluorination reactions. It can be made anhydrous typically by heating it around 40 °C under vacuum.....
Mar 16,2021SurfactantCarbomer
9007-20-9You may like
- Carbomer 940
-

- $0.00 / 20KG
- 2025-04-24
- CAS:9007-20-9
- Min. Order: 1KG
- Purity: 40000-60000
- Supply Ability: 20TONS
- Carbopol
-

- $20.00 / 1kg
- 2025-04-24
- CAS:9007-20-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000
- Carbomer940
-

- $0.00 / 1kg
- 2025-04-24
- CAS:9007-20-9
- Min. Order: 1kg
- Purity: 56.0%-68.0%
- Supply Ability: 1000 KGS






