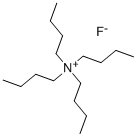
What is Tetrabutylammonium fluoride?
Mar 16,2021
Identification
Product Name: Tetrabutylammonium fluoride
CAS: 429-41-4
MF: C16H36FN
MW: 261.46
EINECS: 207-057-2
Molecular Structure 
Properties
Melting point 62-63 °C(lit.)
density 0.953 g/mL at 25 °C
refractive index n20/D 1.456
Fp 1 °F
storage temp. 2-8°C
solubility Miscible with terahydrofuran, acetonitrile, dimethyl sulfoxide and organic solvents.
form Solution
color Clear light greenish to brown
Water Solubility Insoluble in water.
Tetrabutylammonium fluoride hydrate is known to be a source of F− anion, used in nucleophilic fluorination reactions. It can be made anhydrous typically by heating it around 40 °C under vacuum.
Application
Tetrabutylammonium fluoride is a reagent in organic syntheses in addition, condensation, base-catalyzed cyclization reactions, fluorination and desulfonylation reactions and as a deprotecting agent. Typically dried prior to use. It is a catalyst for etherification of alcohols and phenols with alkyl halides Catalyst for benzylation reactions.
Tetrabutylammonium Fluoride is a reactant used for the synthesis of multiple compounds, including conjugated dienoic acid esters, oligoribonucleotides with phosphonate-modified linkages, and triple monoamine reuptake inhibitors, among others. It has proven useful in studying cancer, diabetes, and neurochemistry.
It is the reactant for preparation of:
• Double clathrate hydrates at high pressures
• Cellulose ethers
• Terminal olefins via dehydrohalogenation reactions
• Neutral and zwitterionic 3-carboranyl thymidine analogues for boron neutron capture therapy
Tetrabutylammonium fluoride hydrate can be used:
• To prepare 2,7-diethynyl-9-propyl-9H-carbazole, which is a key intermediate for the synthesis of calix[4]arene−carbazole polymers.
• As an anion source in the study of selective detection of F− by anion receptor viz Schiff base.
• To prepare terminal olefins from primary alkyl iodides.
- Related articles
- Related Qustion
Poly(acrylic acid) (PAA) is a polymer of acrylic acid, which has a carboxylic group on each monomer unit. This polymer becomes a polyelectrolyte in water via dissociation of the acid groups.....
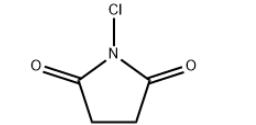
Mar 12,2021Catalyst and AuxiliaryN-Chlorosuccinimide (NCS) is a chlorinating and oxidizing reagent. It is also used as a mild oxidant. NCS is related to succinimide, but with NCl in place of NH. The N-Cl bond is highly reactive, and NCS functions as a source of "Cl+".....
Mar 25,2021Organic reagentsTetrabutylammonium fluoride
429-41-4You may like
Tetrabutylammonium fluoride manufacturers
- Tetrabutylammonium fluoride
-
- $1.00 / 1KG
- 2025-04-23
- CAS:429-41-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Tetranbutylammoniumfluoride
-

- $70.00 / 1kg
- 2025-04-15
- CAS:429-41-4
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000
- Tetrabutylammonium fluoride
-

- $60.00 / 1kg
- 2025-04-15
- CAS:429-41-4
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000