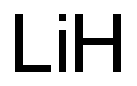What is the oxidation state of Li?
Mar 20,2024
The oxidation state of Lithium (Li) is 0, and the oxidation state of Li+ is +1. Oxidation numberThe oxidation number is a positive or negative number assigned to an atom to indicate its degree of oxidation or reduction. The term oxidation state is often used interchangeably with oxidation number. It is determined according to a set of rules based on the fact that electron pairs in covalent bonds belong exclusively to the more electronegative element. In most compounds, lithium has an oxidation number of +1 because it tends to lose an electron in order to achieve a stable electronic configuration.

- Related articles
- Related Qustion
- Lithium:Chemistry,Reactions,Uses Feb 23, 2023
Lithium is a malleable, soft silvery metal and the first element belonging to the alkali metals. It is small and the lightest among all metals. Its chemistry and that of the rest of the alkali metals is attributed mostly to the ease of invo
- PHYSICAL PROPERTIES OF LITHIUM METAL Dec 16, 2019
Davy and Brande independently prepared lithium metal in 1818 by the electrolysis of lithium oxide. However, larger quantities of the metal were prepared by Bunsen and Mattiessen in 1855 by electrolysis of the chloride. Guntz first proposed
Perfluorotributylamine, valued for stability in electronics and tumor treatment, poses environmental and health risks requiring regulatory control and alternative exploration.....
Nov 13,2024APIThe commercial processes for isoprene production include the extraction method (recovery from C5 fraction), dehydrogenation of isopentane or isopentene, the reaction of isobutylene with formaldehyde, and the acetylene–acetone route.....
Mar 20,2024Organic Chemistry