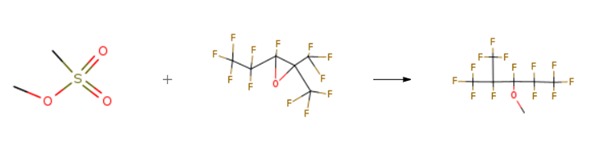
1,1,1,2,2,3,4,5,5,5-Decafluoro-3-methoxy-4-(trifluoromethyl)pentane is used in preparation method of isolated Hydrofluoroether.
1,1,1,2,3,4,4,5,5,5,-Decafluoro-3-methoxy-2-(trifloromethyl)pentane is prepared by the reaction of Methyl methanesulfonate and perfluoro-2,3-epoxy-2-methylpentane. The specific synthesis steps are as follows:
(1). Clean and dry the 500ml reaction flask first; (2). Add 200mL of diethylene glycol dimethyl ether to the flask, turn on the stirring, and add 58g (1mol) of anhydrous potassium fluoride and 52.86g (0.2mol) of 18-crown-6 under stirring; (3). Stir for 10 minutes to fully dissolve anhydrous potassium fluoride and 18-crown ether-6, then add 158g (0.5mol) of perfluoro-2-methyl 2,3-epoxypentane to obtain a solution; (4). Heat the solution water bath to 50°C, keep the temperature constant at about 50°C, slowly add 71.59g (0.65mol) methyl methanesulfonate dropwise with a dropping funnel; (5). Control the dropping time of methyl methanesulfonate for about 2h. After the dropping, the solution will continue to be kept at a constant temperature of 50°C for 3h; (6). After the reaction is completed, cool to room temperature, and then take out the mixed liquid for rectification to obtain the product. Result: The mass of hydrofluoroether obtained is 164.22g, of which 1,1,1,2,2,3,4,5,5,5-decafluoro-3-methoxy-4-(trifluoromethyl)-pentane The content is 96.79%, the residue of perfluoro-2-methyl 2,3-epoxypentane is 0.07%, the conversion rate of perfluoro-2-methyl 2,3-epoxypentane is 99.93%, 1,1,1 The yield of 2,2,3,4,5,5,5-decafluoro-3-methoxy-4-(trifluoromethyl)-pentane was 90.83%.