N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE is an amine organic compound, which can be used as intermediate in organic synthesis.
Add reactant (0.2 mmol, 1.0 equiv), THF (2.0 mL) and CH2Br2 (0.6 mmol, 3.0 equiv) to a flame-dried 10 mL Schlenk tube in a glove box. Seal and take out of the glove box. Cool the reaction mixture to -78°C. Add nBuLi (0.56 mmol, 2.8 equiv) dropwise under N2 atmosphere within 3minutes. Stir the reaction at -78°C for 30 minutes and add ZnCl2 (0.1 mL, 0.5equiv, 1.0 M in Et2O). Allow the mixture to warm to room temperature and stir for 1 hour. Cool the mixture to 0°C. Add a premixture of H2O2 (30% in H2O, 0.5 mL) and NaOH (2.0 M, 1.0 mL). Stir the mixture at room temperature for another 1 hour and dilute with water (20 mL). Extract with DCM (30 mL x 2) and dry over Na2SO4. Filter and concentrate under vacuum. Purify the crude product by silica gel flash column chromatography to obtain product. 1H NMR (CDCl3, 500 MHz) δ 7.08 (d, J = 8.4 Hz, 2H), 6.78 (d, J = 8.4 Hz,2H), 3.80 (t, J = 5.6 Hz, 2H), 3.43 (t, J = 5.4 Hz, 2H), 2.93 (s, 3H), 2.28 (s, 3H), 2.01 (brs, 1H). 13C NMR (CDCl3, 125 MHz) δ 148.3, 129.8, 127.1, 114.0, 60.1, 56.2, 39.1, 20.4.
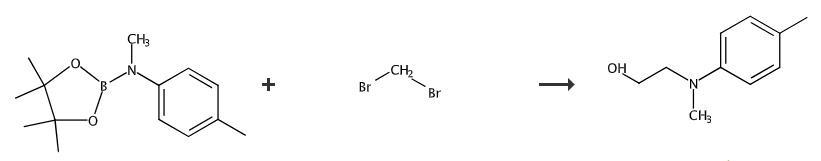
Fig The synthetic method of N-(2-hydroxyethyl)-N-methyl-4-toluidine