Метил-трет-бутиловый эфир химические свойства, назначение, производство
Химические свойства
tert-Butyl methyl ether, also known as MTBE or Methyl tert-butyl ether, is a clear, colorless liquid with a low viscosity that is combustible and has a distinct, turpentine-like odor. It is miscible with organic solvents, but only slightly soluble in water. Methyl tert-butyl ether is very stable under alkaline, neutral, and weakly acidic conditions. In the presence of strong acids, it is cleaved to methanol and isobutene. Depending on reaction conditions the latter can form isobutene oligomers. MTBE does not undergo autoxidation and, in contrast to other ethers, it does not form peroxides with atmospheric oxygen. It improves the antiknock properties when added to motor gasoline.
История
tert-Butyl methyl ether was first synthesized (by the classical Williamson ether synthesis) and characterized in 1904. Extensive studies in the United States during World War II demonstrated the outstanding qualities of MTBE as a high-octane fuel component. It was first commercially produced in Italy in 1973 for use as an octane enhancer in gasoline. U.S. production of MTBE started in 1979 after Atlantic Richfield Co. (ARCO) was granted a waiver by the U.S. Environmental Protection Agency (EPA) that allowed MTBE to be blended up to 7 vol % in U.S. unleaded gasoline. The use of other aliphatic ethers was allowed when the U.S. EPA issued its “substantially similar” definition for unleaded gasoline specifications in 1981. Under this definition, any aliphatic ether or ether mixture could be blended in unleaded gasoline as long as the total oxygen contribution from the ethers does not exceed 2.0% oxygen by weight in the gasoline. This allowed MTBE to be blended up to approximately 11 vol % in gasoline.
Использование
Methyl tert-butyl ether (MTBE) was primarily used as a gasoline additive in unleaded gasoline in the United States prior to 2005, in the manufacture of isobutene, and as a chromatographic eluent especially in high pressure liquid chromatography. It is also a pharmaceutical agent and can be injected into the gallbladder to dissolve gallstones (ATSDR, 1996). tert-Butyl methyl ether is also used in the petrochemical industry. By reversal of its formation reaction, MTBE can be cracked to isobutene and methanol on acidic catalysts at higher temperature than MTBE synthesis.
Подготовка
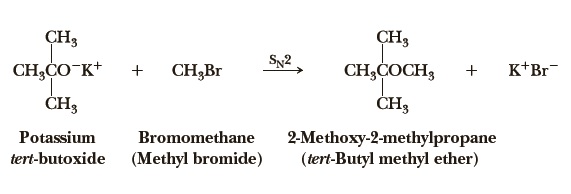
tert-butyl methyl ether can be prepared by the reaction of potassium tert-butoxide and bromomethane. Methyl tert-butyl ether also can be obtained by the acid-catalyzed addition of methanol to isobutene. Suitable catalysts are solid acids such as bentonites, zeolites and – commonly used in industrial world scale MTBE-production units – macroporous acidic ion-exchange resins. The reaction is weakly exothermic with a heat of reaction of -37.7 kJ/mol.
Определение
ChEBI: Methyl tert-butyl ether is an ether having methyl and tert-butyl as the two alkyl components. It has a role as a non-polar solvent, a fuel additive and a metabolite.
Общее описание
Methyl tert-butyl ether appears as a colorless liquid with a distinctive anesthetic-like odor. Vapors are heavier than air and narcotic (cause drowsiness when inhaled). This liquid has a flash point lower than most ambient temperatures, so it will readily ignite under most conditions. It is less dense than water and moderately soluble in water. Used as a octane booster in gasoline.
Реакции воздуха и воды
Highly flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979. p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously [MCA Case History 616. 1960].
Профиль реактивности
Tert-Butyl methyl ether is a kind of Ethers, it form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.
Опасность
Tert-Butyl methyl ether is slightly toxic by ingestion and inhalation. Flammable when exposed to heat or flame. Upper respiratory tract irritant and kidney damage. Questionable carcinogen.
Угроза здоровью
INHALATION: May cause dizziness or suffocation. Contact may irritate or burn eyes or skin. May be harmful if swallowed.
Промышленное использование
Methyl tert-butyl ether (MTBE) is used as an octane enhancer in gasoline. EPA
regulations allow up to 2.7 wt.% oxygen in gasoline which allows 15 vol.%
MTBE in gasoline. Other alkyl ethers can also be blended into gasoline up to the
2.7 wt% oxygen requirement. The stability of MTBE to oxidation and peroxide
formation gives this unsymmetrical ether an advantage over other ethers in various
extraction and reaction solvent applications.
Профиль безопасности
Poison by intravenous
route. Slightly toxic by ingestion and
inhalation. Flammable when exposed to heat
or flame. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also ETHERS.
Возможный контакт
Tert-Butyl methyl ether is used as an organic solvent; as an
octane booster in unleaded gasolines; in making other chemicals; and in medicine to dissolve gall stones
Описание
Метил-трет-бутиловый эфир (МТБЭ) является кислородсодержащим октаноповышающим компонентом и применяется для повышения октанового числа автобензинов.
Химические свойства
По исследовательскому методу МТБЭ характеризуется высокими октановыми числами – 115-135, по моторному методу – 98-100. Растворяется только в бензине, не ядовит.
МТБЭ растворим в этаноле, диэтиловом эфире, плохо — в воде (4,6% при 20˚С)
Образует азеотропные смеси:
— с метанолом (МТБЭ — 85% мас.), температура кипения – 52˚C;
— с водой (МТБЭ — 96%мас.), температура кипения — 52,6˚C.
При высоких температурах (460˚C) или использовании катализатора происходит разложение на метанол и изобутилен.
Кроме МТБЭ во всем мире имеет место применение и других топливных оксигенатов.
Канцерогенность
EPA has not classified methyl tert-butyl ether with respect to potential carcinogenicity. There is evidence for the carcinogenicity of MTBE in animals. MTBE causes leukemias/lymphomas in female rats, renal tubular tumors and Leydig cell tumors in male rats, and hepatocellular tumors in mice. Positive animal carcinogenicity data and some further concordance in tumor sites for formaldehyde and TBA, metabolites of MTBE, provide support for this conclusion. However, uncertainties remain about the nature and extent of risk at very low doses, and about the particular tumor sites that are most relevant to humans.
Экологическая судьба
tert-Butyl Methyl Ether can be released during manufacturing or blending with gasoline; during the storage, distribution, and transfer of MTBE-blended gasoline; and from spills or leaks or fugitive emissions at automotive service stations (U.S. EPA, 1994a). Vapor emissions from MTBE-blended gasoline may also contribute to atmospheric levels (U.S. EPA, 1988). It is not expected to persist in the atmosphere because it undergoes destruction from reactions with hydroxyl radicals. A total atmospheric lifetime for MTBE of approximately 3 and 6.1 days has been reported in polluted urban air and in nonpolluted rural air, respectively (U.S. EPA, 1993a). Based upon its vapor pressure and Henry s law constant, MTBE is highly volatile and would be expected to evaporate rapidly from soil surfaces or water. It may be fairly persistent when introduced into subsurface soils or to groundwater since volatilization to the atmosphere is reduced or eliminated. It does not readily degrade in surface waters due to hydrolysis or other abiotic processes. It is also resistant to biodegradation (U.S. EPA, 1993a). It is usually removed from surface waters very rapidly because of its high volatility. If released as part of a gasoline mixture from leaking underground storage tanks, its relatively high water solubility combined with little tendency to sorb to soil particles encourages migration to local groundwater supplies (U.S. EPA, 1993a).
Производство
Получение Метил-трет-бутиловый эфир основано на простой одностадийной технологии присоединения метилового спирта CH3OH к изобутилену (2-метилпропену) C4H8
без воздействия высоких температур и давлений. Протекание реакции в
специальном катализаторе, чаще с применением ионообменных смол,
обеспечивает полную конверсию и высокую селективность, где сырьем
является фракция С4 каталитического крекинга с присутствием изобутилена и н-бутилена (1- и 2-бутены) C4H8.
Перевозки
UN2398 Methyl tert-butyl ether, Hazard Class: 3;
Labels: 3-Flammable liquid.
Методы очистки
Purify as for n-butyl methyl ether. [Beilstein 1 IV 1615.]
Несовместимости
May form explosive mixture with air.
May be able to form unstable peroxides. Much less likely
to form peroxides than other ethers. Incompatible with
strong acids. Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. May accumulate static
electrical charges, and cause ignition of its vapors.
Утилизация отходов
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be observed.
Метил-трет-бутиловый эфир препаратная продукция и сырье
сырьё
препарат
Methyl 2-aminothiophene-3-carboxylate
(1R,2R)-(-)-1,2-диаминоциклогексан
1-[(трет-бутоксикарбонил)амино]циклопропанкарбоновая кислота
(-)-2-азабицикло[2.2.1]гепт-5-ен-3-он
Этил 2-(бромметил)акрила
5-МЕТОКСИ-1H-ПИРРОЛО[3,2-B]ПИРИДИН-2-КАРБОНОВАЯ КИСЛОТА
2,2,2-Трихлорэтил хлорформиат
BIS(4-NITROBENZYL) PHOSPHOROCHLORIDATE
Трис(дибензилиденацетон)дипалладий-хлорформ аддук
3-N-Boc-amino-azetidine
4-Винилбензилхлорид
N,N-Diethylacetoacetamide
Натрия триацетоксиборгидрид
Бринзоламид
5-аминоиндол гидрохлорид
4-Aminotetrahydropyran hydrochloride
N-метил-p-анизидин
3-AMINO-ISONICOTINIC ACID ETHYL ESTER
2-хлормалональдегид
Азетидин
4-PYRIDIN-2-YLISOXAZOL-5-AMINE
2-(2-НИТРОФЕНИЛ)АКРИЛАЛДЕГИД
(3-AMINO-PYRIDIN-4-YL)-METHANOL
Тетрагептиламмоний бромид
Тетракис (трифенилфосфин) палладий
5-МЕТОКСИ-1H-ПИРРОЛО [2,3-C] ПИРИДИН-2-КАРБАЛЬДЕГИД
1-амино-1-циклопропанкарбоновой кислоты
5-METHOXY-1H-PYRROLO[2,3-C]PYRIDINE-2-CARBOXYLIC ACID
н-Octadecyltrichlorosilane
6-Нитроиндол
N-Phospho-L-arginine
Трет.-бутил метил малона
1,1,1,5,5,5-гексафторацетилацетон
6-аминоиндол
Азетидин гидрохлорид
3-метоксибензил бромид
4,6-Dimethylpyrimidine-5-carboxylic acid
2-МЕТОКСИЭТИЛАЦЕТАТ
3-аминопиридин-4-карбоксальдегида
ФОСФО-L-АРГИНИН НАТРИЯ