Dolutegravir sodium химические свойства, назначение, производство
Описание
Dolutegravir, also known as DTG or dolutegravir sodium, is an antiretroviral therapy drug used to treat HIV infection. It belongs to the Integrase Strand Transfer inhibitor (INSTi) class of drugs and was fast-tracked by the FDA in February 2012. GlaxoSmithKline developed and markets dolutegravir sodium (Tivicay), which received FDA approval in August 2013 as a novel integrase inhibitor for HIV treatment, including adults undergoing their first treatment as well as those who have been treated with other integrase transfer strand inhibiting agents.
Использование
Dolutegravir, a second-generation HIV-1 integrase strand transfer inhibitor, is commonly used along with other medications to manage HIV infection. Its potency in inhibiting HIV replication has been demonstrated in various cell types infected with a self-inactivating PHIV lentiviral vector, including peripheral blood mononuclear cells (PBMCs), MT-4 cells, and CIP4 cells.
Определение
ChEBI: Dolutegravir sodium is an organic sodium salt that is the monosodium salt of dolutegravir. Used for treatment of HIV-1. It has a role as a HIV-1 integrase inhibitor. It contains a dolutegravir(1-).
Побочные эффекты
Dolutegravir, an HIV medication, can lead to a variety of side effects. While some can be serious, many, such as nausea or sporadic dizziness, can be effectively managed. Dolutegravir may also cause alterations in your immune system, resulting in a condition known as immune reconstitution inflammatory syndrome (IRIS).
clinicalinfo.hiv.gov/en/drugs/dolutegravir/patientСинтез
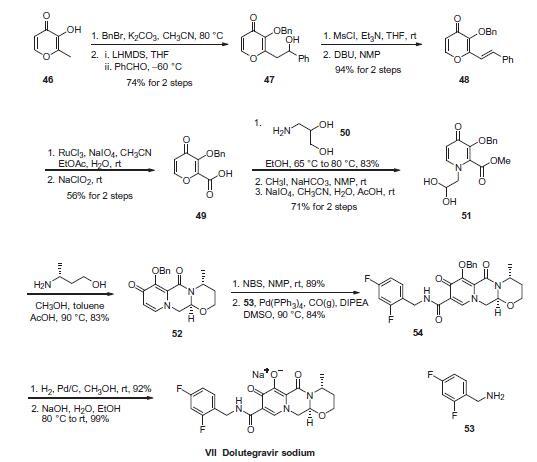
The most likely process-scale synthesis of dolutegravir sodium,
began with benzyl protection and alkylation
of pyrone 46 with benzaldehyde, yielding alcohol 47 in 74% over 2 steps. Alcohol mesylation and in situ elimination
provided the styrenyl olefin 48 in 94% yield, which further
underwent an oxidative cleavage of the olefin to generate 49 by
sequential addition of RuCl3/NaIO4 and NaClO2 (56% overall yield).
Treatment of pyranone 49 with 3-amino-propane-2-diol (50) in
ethanol at elevated temperatures delivered the corresponding
pyridinone in 83% yield, and this was followed by esterification
and sodium periodate-mediated diol cleavage to furnish
intermediate 51 in 71% overall yield across the two-step
sequence. l Next, the key ring-forming step in the synthesis
of dolutegravir sodium consisted of cyclization of 51 with (R)-3-
amino-butan-1-ol, a process which relies on substrate control to
provide the desired tricyclic carbamoylpyridone system 52 in high
stereoselectivity (20/1 in favor of the desired isomer).51 Previously,
cyclization of systems such as 51 with unsubstituted amino alcohols
were found to yield a mixture of diastereomeric products,
therefore indicating the pivotal role of the chiral amino alcohol
in influencing stereochemical bias during the overall cyclization
step. In practice, reaction of 51 with (R)-3-amino-butan-1-ol
at 90 ?? led to isolation of a single cyclization product 52, after
recrystallization from EtOAc. From 52, N-bromosuccinimide
(NBS) bromination and subsequent treatment with amine 53 under
palladium-catalyzed amidocarbonylative conditions led to amide
54 in 75% yield over 2 steps. Finally, removal of the benzyl group
and subsequent crystallization using sodium hydroxide in water
and ethanol provided dolutegravir sodium (VII) in 99% yield.
Dolutegravir sodium препаратная продукция и сырье
сырьё
O-Methyl Dolutegravir
(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide
препарат