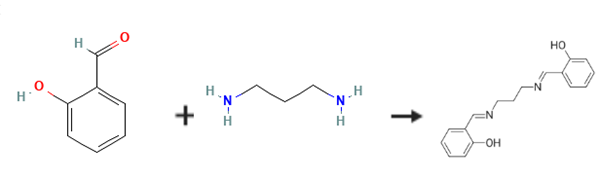
N,N'-Bis(salicylidene)-1,3-propanediamine is prepared by the reaction of 2-Hydroxybenzaldehyde and 1,3-diaminopropane. The specific synthesis steps are as follows:
General procedure: This Schiff base was prepared via a condensation reaction in EtOH under hydrothermal conditions using 2-hydroxy-benzaldehyde and 1,3-diaminopropane. 2-Hydroxy-benzaldehyde (0.02 mol, 2.44 g) was dissolved in 40 cm3 of warm EtOH, then 0.01 mol (0.74 g) of 1,3-diaminopropane was added to this solution and heated up to the boiling point. After cooling, yellow crystals were filtered and air-dried. Yield: 92-95%, mp 58 °C (determined by TG). Elemental Anal. Calc. for C17H18N2O2: C, 72.3; H, 6.43; N, 9.92. Found: C, 71.9; H, 6.45; N, 10.47%. λmax = 243 nm, ε = 7045 dm3 mol-1 cm-1 in DMSO, λmax = 242 nm, ε = 7865 dm3 mol-1 cm-1 in MeOH. IR (cm-1): νO-H 2627, νC-H(Ar) 3021-3019, νC-H(aliph) 2929-2862, νC=N 1629, νC=C(ring) 1608, νC-O(phenol) 1274-1151, δC-H(Ar) 762. 1H NMR in d6-DMSO: 13.51 (s) (O-H), 8.60 (s) (-CH=), 7.43 (d) (HAr), 7.32 (t) (HAr), 6.88 (t) (HAr), 3.68 (t) (N-CH2-), 2.01 (p) (-CH2-). 13C NMR in d6-DMSO: 166.6, 161.1, 132.7, 132.1, 119.1, 118.9 (CAr), 116.9 (-C=N), 58.5 (N-CH2-), 31.9 (-CH2-). m/z: 282 [M]+, 161 [HO-C6H4-CH=N-CH2-CH2-CH2]+, 148 [HO-C6H4-CH=N-CH2-CH2]+ (BP), 134 [HO-C6H4-CH=N-CH2]+, 120 [HO-C6H4-CH=N]+, 107 [HO-C6H4-CH2]+, 77 [C6H5]+.