ТРИФТОРИД АЗОТА химические свойства, назначение, производство
Описание
Nitrogen trifluoride is a colorless gas with little odor. Nitrogen trifluoride is an oxidizer that is thennodynamically stable except at elevated temperatures. At temperatures up to about 482°F (250°C), its reactivity is comparable to oxygen. At higher temperatures, its reactivity is similar to fluorine owing to appreciable dissociation into NF2 and F-. The thennal dissociation of nitrogen trifluoride has been studied by a number of investigators and has been found to peak in the temperature range of 1100K to 1500K. In handling nitrogen trifluoride, conditions should be avoided that can result in high temperatures such as adiabatic compression from the rapid pressurization of a system.
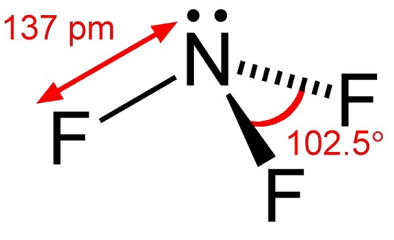
Nitrogen trifluoride acts primarily upon the elements as a fluorinating agent, but not a very active one at lower temperatures. At elevated temperatures, nitrogen trifluoride pyrolyzes with many of the elements to produce nitrogen tetrafluoride and the corresponding fluoride. The pyrolysis of nitrogen trifluoride over copper turnings produces nitrogen tetrafluoride in a 62 percent to 71 percent yield at 707°F (375°C). Pyrolysis over carbon is more complete.
Химические свойства
Nitrogen trifluoride is a colorless gas. Moldy
odor. Shipped as a nonliquefied compressed gas.
Физические свойства
Colorless gas; moldy odor; liquefies at -128.75°C; density of liquid 3.116 g/mL; vapor pressure at -158°C 96 torr; solidifies at -206.8°C; critical temperature -39.15°C; critical pressure 44.02 atm; critical volume 126 cm3/mol; very slightly soluble in water.
Использование
Nitrogen trifluoride is an etchant and chamber cleaning agent.
Oxidizer for high-energy fuels, chemical synthesis.
Подготовка
Nitrogen trifluoride is prepared by electrolysis of either molten ammonium fluoride, NH4F, or melted ammonium acid fluoride, NH4HF2 (or ammonium fluoride in anhydrous HF). While the NH4F method is preferred because it forms nitrogen trifluoride as the only product, electrolysis of ammonium acid fluoride yields a small amount of dinitrogen difluoride, N2F2,and NF3.
Also, nitrogen trifluoride can be prepared by reaction of ammonia with fluorine diluted with nitrogen in a reactor packed with copper. Other nitrogen fluorides, such as N2F2, N2F4, and NHF2 also are produced. The yield of major product depends on fluorine/ammonia ratio and other conditions.
Методы производства
Nitrogen trifluoride can be formed from a wide variety of chemical reactions. The commercial process for production involves direct fluorination of ammonia with fluorine gas in the presence ofammonium fluoride.
Реакции
Hydrogen reacts with nitrogen trifluoride with the rapid liberation of large amounts of heat and is the basis for the use of nitrogen trifluoride in high-energy chemical lasers. The flammability range for nitrogen trifluoride-hydrogen mixtures is 9.4 mole percent to 95 mole percent nitrogen trifluoride. Nitrogen trifluoride reacts with organic compounds, but generally an elevated temperature is required to initiate the reaction. Under these conditions, the reaction will often proceed explosively, and great care must be exercised when exposing nitrogen trifluoride to organic compounds. Therefore, nitrogen trifluoride has found little use as a fluorinating agent for organic compounds.
Общее описание
A colorless gas with a moldy odor. Very toxic by inhalation. Slightly soluble in water. Corrosive to tissue. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Used to make other chemicals and as a component of rocket fuels.
Реакции воздуха и воды
Slightly soluble in water.
Профиль реактивности
Nitrogen trifluoride is a very powerful oxidizing agent. Presents dangerous fire hazard in the presence of reducing agents. Etches glass in the presence of moisture. Emits toxic and corrosive fumes of fluoride when heated to decomposition [Lewis, 3rd ed., 1993, p. 937]. Can react violently with hydrogen, ammonia, carbon monoxide, diborane, hydrogen sulfide, methane, tetrafluorohydrazine, charcoal. Explosive reaction with chlorine dioxide. A severe explosion may occur when exposed to reducing agents under pressure [Bretherick, 5th ed., 1995, p. 1427].
Опасность
Severe explosion hazard. Corrosive to tissue. Methemoglobinemia, liver and kidney damage.
Угроза здоровью
Inhaling nitrogen trifluoride can reduce the capacity of red blood cells to carry oxygen. This causes cyanosis, or a bluish discoloration of the skin. Breathing nitrogen trifluoride can also lead to headache, dizziness, weakness and confusion. After prolonged exposure to high concentrations, breakdown of red blood cells and changes in the liver, kidneys, spleen and heart muscle may occur as secondary effects. In fresh air, the initial red blood cell changes will clear over several hours, but the person should still be monitored for secondary effects.
Пожароопасность
Substance does not burn but will support combustion. Some may react explosively with fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Vapors from liquefied gas are initially heavier than air and spread along ground. Runoff may create fire or explosion hazard. Containers may explode when heated. Ruptured cylinders may rocket.
Промышленное использование
Nitrogen trifluoride has been used successfully in large quantities as a fluorine source for high-energy chemical lasers. It is preferred over fluorine because of its comparative ease of handling at ambient conditions.
Recently, an increasing amount of nitrogen trifluoride is being used in the semiconductor industry as a dry etchant, showing significantly higher etch rates and selectivities when compared to carbon tetrafluoride and mixtures of carbon tetrafluoride and oxygen.
Nitrogen trifluoride was also used as an oxidizer in rocketry in the early 1960s, but this application was not commercialized.
Использование в материалах
At temperatures less than 482°F (250°C), nitro�gen trifluoride has a reactivity similar to that of
oxygen and is relatively inert to most materials
of construction. At ambient temperatures, brass,
aluminum, copper, steel, and stainless steels can
be used because corrosion rates of less than 0.1
mil/yr. at 160°F (71.1°C) have been determined
for these materials. Nitrogen trifluoride is also
compatible with fluorinated materials such as
Teflon at ambient temperatures.
At increased temperatures and pressures, ni�trogen trifluoride's reactivity increases becom�ing more like that of fluorine, with nickel and
Monel being the preferred materials of con�struction.
Профиль безопасности
A poison. Mildly toxic
by inhalation. Prolonged absorption may
cause mottling of teeth, skeletal changes.
Severe explosion hazard by chemical
reaction with reducing agents, particularly
when under pressure. A very dangerous fire
hazard; a very powerful oxidner; otherwise
inert at normal temperatures and pressures.
Возможный контакт
This material has been used in chemical
synthesis and as an oxidizer for high-energy fuels (as
an oxidizer in rocket propellant combinations).
хранилище
Nitrogen trifluoride cylinders must be securely supported while in use to prevent movement and straining of connections. Full cylinders must be stored in a well-ventilated area, protected from excessive heat (125°F or 51.7°C), located away from organic or flammable materials, and secured. Valve protection caps and valve outlet caps must be securely in place at all times when the cylinder is not in use.
Перевозки
UN2451 Nitrogen trifluoride, Hazard Class: 2.2;
Labels: 2.2-Nonflammable compressed gas; 5.1-Oxidizer.
Cylinders must be transported in a secure upright position,
in a well-ventilated truck. Protect cylinder and labels from
physical damage. The owner of the compressed gas cylinder
is the only entity allowed by federal law (49CFR) to
transport and refill them. It is a violation of transportation
regulations to refill compressed gas cylinders without the
express written permission of the owner.
Несовместимости
The gas is a powerful oxidizer. Presents
dangerous fire hazard in the presence of reducing agents. Etches glass in the presence of moisture. Reacts with
oil, grease, reducing agents and other oxidizable materials;
combustibles, organics, ammonia, carbon monoxide; methane,
hydrogen, hydrogen sulfide; activated charcoal; diborane,
water. Can react violently with hydrogen, ammonia,
carbon monoxide, diborane, hydrogen sulfide, methane, tetrafluorohydrazine,
charcoal. Nitrogen trifluoride will
increase intensity of an existing fire.
Утилизация отходов
Return refillable compressed
gas cylinders to supplier. Vent into large volume of concentrated
reducing agent (bisulfites, ferrous salts or hypo)
solution, then neutralize and flush to sewer with large
volumes of water.
ТРИФТОРИД АЗОТА препаратная продукция и сырье
сырьё
препарат