Омепразол химические свойства, назначение, производство
Описание
Omeprazole is a potent gastric antisecretory agent with selective inhibitory effect on the
H+,K+-ATPase proton pump. It is highly effective in the treatment of duodenal ulcer and
Zollinger-Ellison syndrome, and is reportedly superior to ranitidine in the management of
reflux esophagitis.
Химические свойства
White Crystalline Solid
Использование
Omeprazole is a proton pump inhibitor used to treat diseases like gastroesophageal reflux disease (GERD), used for gastric and duodenal ulcers, reflux or erosive esophagitis, and Zollinger-Ellison syndrome. It is also effective for gastric and duodenal ulcers that are ineffective with H2 receptor antagonists. Injections of Omeprazole can also be used for: 1 gastrointestinal bleeding, such as peptic and anastomic ulcer bleeding, and the prevention of severe diseases (such as cerebral hemorrhage, severe trauma, etc.) and gastric surgery caused by upper intestinal bleeding; 2 acute gastric mucosal damage complicated by stress or nonsteroidal anti-inflammatory drugs; 3 general anesthesia, post-surgery, or coma patients, to prevent acid reflux and aspiration pneumonia; 4 Combined with amoxicillin and clarithromycin, or with metronidazole and clarithromycin, it can effectively kill Helicobacter pylori (Hp).
прикладной
Omeprazole is a benzimidazole with selective and irreversible proton pump inhibition activity. Omeprazole forms a stable disulfide bond with the sulfhydryl group of the hydrogen-potassium (H+ - K+) ATPase found on the secretory surface of parietal cells, thereby inhibiting the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen and suppressing gastric acid secretion. This agent exhibits no anticholinergic activities and does not antagonize histamine H2 receptors. Omeprazole Pellets are used in the treatment of Gastroesophageal reflux disease (GERD): A condition in which backward flow of acid from the stomach causes heartburn and injury of the food pipe (esophagus).
Определение
ChEBI: Omeprazole is a member of the class of benzimidazoles that is 1H-benzimidazole which is substituted by a [4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl group at position 2 and a methoxy group at position 5.
Подготовка
The antiulcer agent omeprazole is produced from 2,3,5-trimethylpyridine N-oxide.
Synthesis and Structure of Omeprazole
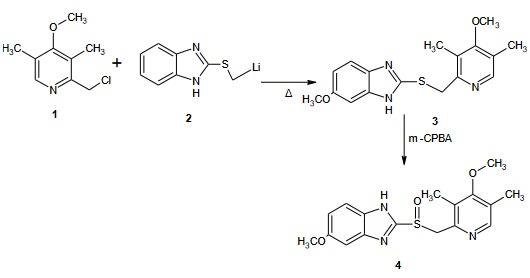
Steps: 2-(Lithium methyl sulphinyl)-5-methoxy-1H benzimidazole 20g was reacted with 2-chloro-3,5-dimethyl-4-methoxy pyridine 21 g to form sulphide intermediate and then converted to Omeprazole when treated with m-CPBA which used as anoxidizingagents. The acetamide-sulfide compounds modification are oxidised to form the amide sulfinyl compound and gives the sulfinyl carboxylate or salts upon alkaline hydrolysis.On further decarboxylation leads to the target molecules. The residual, unreacted salt, inorganic by-products and other minor by-products can be easily purified by a simple washing from omeprazole or lansoprazole. The amide compounds containing crystalline solids as opposed to the sulphide and sulfoxides of the reported procedures.
DOI: http://dx.doi.org/10.20902/IJPTR.2019.120307
Всемирная организация здравоохранения(ВОЗ)
Omeprazole was introduced in the 1980s. It belongs to a group of
agents that have an inhibitory effect on the secretion of hydrochloric acid in the
stomach (gastric acid proton pump inhibitors) and is used in the treatment of
upper gastrointestinal tract disorders. The Committee for Proprietary Medicinal
Products of the European Commission has concluded that a causal association
between the reactions reported in Germany and the use of omeprazole had not
been established. Nevertheless oral administration should be preferred.
(Reference: (CPMPPO) Pharmacovigilance Opinion, No.16 , , 25 July 1994)
Общее описание
Omeprazole, 5-methoxy-2-(((4-methoxy-3, 5-dimethyl-2-pyridinyl)methyl) sulfinyl)-1Hbenzimidazole(Losec), is a white to off-white crystallinepowder with very slight solubility in water. Omeprazole isan amphoteric compound (pyridine N, pKa 4.06; benzimidazoleN-H, pKa 0.79), and consistent with the proposedmechanism of action of the substituted benzimidazoles, isacid labile. Hence, the omeprazole product is formulatedas delayed-release capsules containing enteric-coatedgranules.
The absolute bioavailability of orally administeredorneprazole is 30% to 40% related to substantial first-passbiotransformation. The drug has a plasmahalf-life of about 1 hour. Most (77%) of an oral dose ofomeprazole is excreted in the urine as metabolites with insignificantantisecretory activity. The primary metabolitesof omeprazole are 5-hydroxyomeprazole (CYP2C19) andomeprazole sulfone (CYP3A4). The antisecretory actions ofomeprazole persist for 24 to 72 hours, long after the drughas disappeared from plasma, which is consistent with itssuggested mechanism of action involving irreversible inhibitionof the proton pump.
Omeprazole is approved for the treatment of heartburn,GERD, duodenal ulcer, erosive esophagitis, gastric ulcer,and pathological hypersecretory conditions.
Биологическая активность
H + ,K + -ATPase inhibitor (IC 50 = 5.8 μ M) that displays antisecretory and antiulcer activity. Inhibits gastric acid secretion (IC 50 = 0.16 μ M for histamine-induced acid formation) and reduces gastric lesion formation induced by a variety of ulcerative stimuli. Antibacteral against Helicobacter pylori in vitro . Also inhibits CYP2C19, CYP2C9 and CYP3A (K i values are 3.1, 40.1 and 84.4 μ M respectively) and blocks swelling-dependent chloride channels (ICIswell).
Клиническое использование
Omeprazole is a proton-pump inhibitor used in the management and treatment of several conditions, including uncomplicated heartburn, peptic ulcer disease, gastrointestinal reflux disease, Zollinger-Ellison syndrome, multiple endocrine adenomas, systemic mastocytosis, erosive esophagitis, gastric ulcers, and helicobacter pylori infection.
Метаболический путь
When male humans are given 14C-omeprazole orally,
an average of 79% of the dose is recovered in the
urine in 96 h. Omeprazole is completely metabolized
and at least six metabolites are identified. Two major
metabolites are hydroxyomeprazole and omeprazole
acid.
Способ действия
Omeprazole is a proton pump inhibitor which can specifically act on gastric parietal cell proton pump sites and transform into the active form of sulfonamide, then irreversibly binds to the proton pumps through disulfide bonds, generating a sulfonamide and proton pump compound (H + -K + -ATP), thereby inhibiting the enzymatic activity, preventing the H+ in parietal cells from being transported to the stomach cavity. It has a strong and persistent inhibitory role on gastric acid secretion caused by basal gastric acid and pentapeptide gastric acid secretions, greatly reducing gastric acid within the gastric juice. Rapid, reversible, and no H2 antagonist-induced psychiatric side effects.
Омепразол препаратная продукция и сырье
сырьё
препарат