МЕТИЛАКРИЛАТ химические свойства, назначение, производство
Описание
Methyl acrylate is an organic compound with the formula CH2CHCO2CH3. It is the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates.
Химические свойства
Methyl acrylate is a clear, colorless, corrosive liquid with a sharp, fruity odor. It is soluble in water and completely miscible with most organic solvents.

Methyl acrylate has a variety of industrial uses. the more important commercial uses of methyl acrylate include the preparation of thermoplastic coatings, use in the manufacture of acrylic and modacrylic fibers. In the fiber application, methyl acrylate is used as a comonomer with acrylonitrile. These acrylic fibers usually contain about 85% acrylonitrile and are used to fabricate clothing, blankets, carpets, and curtains. Other uses of methyl acrylate include coatings, adhesives, textile backcoatings, elastomers, plastics, and it is also found in ionic exchange resins, barrier film resins, antioxidant intermediates and acrylic fibers.
Физические свойства
Clear, colorless, flammable liquid with a heavy, sweet, pungent odor. An odor threshold
concentration of 3.5 ppbv was reported by Nagata and Takeuchi (1990).
Использование
Methyl acrylate is a monomer used in the manufacture of acrylic fibers, plastic films, textiles, papercoatings, and other acrylate ester resins. It is also used in amphoteric surfactants.
Подготовка
Acrylate esters can be produced in a number of ways. The most commonly used method, developed in 1970, involves a propylene oxidation process. The reaction occurs initially with the oxidation of propylene to acrolein, which in turn is oxidized to acrylic acid. Once the acrylic acid is formed, it is reacted with methanol which causes the formation of the methyl acrylate. This reaction is shown as follows:
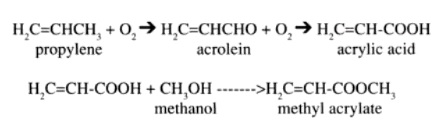
An older method, the Reppe process, involves reacting acetylene with nickel carbonyl and methyl alcohol in the presence of an acid to produce methyl acrylate.
More recent methods for producing acrylate esters involve the use of organic carbonates as esterifying agents or isolating 2-halo- 1-alkenes from hydrocarbon feedstocks to produce the acrylate esters (Haggin, 1985).
Методы производства
Methyl acrylate is manufactured via a reaction of nickel
carbonyl and acetylene with methanol in the presence of
an acid; more commonly, however, it is manufactured
via oxidation of propylene to acrolein and then to
acrylic acid. The acid is reacted with methanol to yield
the ester.
Определение
methacrylate: A salt or ester ofmethacrylic acid (2-methylpropenoicacid).
Общее описание
Colorless volatile liquid with an acrid odor. Flash point 27°F. Vapors may irritate the eyes and respiratory system. Highly toxic by inhalation, ingestion and skin absorption. Less dense than water (0.957 gm / cm3) and slightly soluble in water, hence floats on water. Vapors heavier than air.
Реакции воздуха и воды
Highly flammable. Forms peroxides when exposed to air that may initiate spontaneous, exothermic polymerization. Peroxide formation usually proceeds slowly. Slightly soluble in water.
Профиль реактивности
METHYL ACRYLATE ignites readily when exposed to heat, flame or sparks. Offers a dangerous fire and explosion hazard. Reacts vigorously with strong oxidizing materials. Forms peroxides when exposed to air that may initiate spontaneous exothermic polymerization. Peroxide formation usually proceeds slowly. Added inhibitor retards polymerization. If the inhibitor is consumed during long storage, explosive polymerization may occur [MCA Case History No. 2033]. Also subject to strongly exothermic polymerization if heated for prolonged periods or contaminated.
Опасность
Flammable, dangerous fire and explosion
risk. Toxic by inhalation, ingestion, and skin absorption; irritant to skin, eyes and upper respiratory tract
irritant; eye damage. Questionable carcinogen.
Угроза здоровью
The liquid is a strong irritant, and prolongedcontact with the eyes or skin may causesevere damage. Inhalation of its vapors cancause lacrimation, irritation of respiratorytract, lethargy, and at high concentrations,convulsions. One-hour exposure to a concen tration of 700–750 ppm in air caused deathto rabbits. The oral toxicity of methyl acry late in animals varied from low to moderate,depending on species, the LD50 values ranging between 250 and 850 mg/kg. The liquidmay be absorbed through the skin, producingmild toxic effects.
Пожароопасность
Flammable liquid; flash point (closed cup)
-4°C (25°F), (open cup) -3°C (27°F); vapor
pressure 68 torr at 20°C (68°F); vapor density
3.0 (air = 1); the vapor is heavier than air and
can travel a considerable distance to a source
of ignition and flashback; autoignition tem perature not established; fire-extinguishing
agent: dry chemical, CO2, or “alcohol” foam;
use water to keep the fire-exposed containers
cool and to flush or dilute any spill; the vapors
may polymerize and block the vents.
The vapors of methyl acrylate form explo sive mixtures with air, over a relatively wide
range; the LEL and UEL values are 2.8 and
25.0% by volume in air, respectively. Methyl
acrylate undergoes self-polymerization at
25°C (77°F). The polymerization reaction
proceeds with evolution of heat and the
increased pressure can cause rupture of
closed containers. The reaction rate is accelerated by heat, light, or peroxides. Vigorous
to violent reaction may occur when mixed
with strong oxidizers (especially nitrates and
peroxides) and strong alkalie.
Профиль безопасности
Poison by ingestion and
intraperitoneal routes. Moderately toxic by
skin contact. Mddly toxic by inhalation.
Human systemic effects by inhalation:
olfaction effects, eye effects, and respiratory effects. A skin and eye irritant. Mutation
data reported. Chronic exposure has
produced injury to lungs, liver, and kidneys
in experimental animals. Questionable
carcinogen. Dangerously flammable when
exposed to heat, flame, or oxidzers.
Dangerous explosion hazard in the form of
vapor when exposed to heat, sparks, or
flame. Can react vigorously with oxidzing
materials. A storage hazard; it forms
peroxides, which may initiate exothermic
polymerization. To fight fire, use foam,
COa, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also ESTERS.
Безопасность
It is an acute toxin with an LD50 (rats, oral) of 300 mg/kg and a TLV of 10 ppm.
Возможный контакт
Methyl acrylate is used in production
of acrylates, copolymers, barrier resins; and surfactants for
shampoos; as a monomer in the manufacture of polymers
for plastic films, textiles, paper, and leather coating
resins. It is also used as a pesticide intermediate and in
pharmaceutical manufacture.
Канцерогенность
Methyl acrylate was not shown to be carcinogenic in male and female rats in a lifetime inhalation study. Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.
Экологическая судьба
Photolytic. Polymerizes on standing and is accelerated by heat, light, and peroxides (Windholz
et al., 1983). Methyl acrylate reacts with OH radicals in the atmosphere (296 K) and aqueous
solution at rates of 3.04 x 10-12 and 2.80 x 10-12 cm3/molecule?sec, respectively (Wallington et al.,
1988b).
Chemical/Physical. Begins to polymerize at 80.2 °C (Weast, 1986). Slowly hydrolyzes in water
forming methyl alcohol and acrylic acid (Morrison and Boyd, 1971). Based on a hydrolysis rate
constant of 0.0779/M?h at pH 9 at 25 °C, an estimated half-life of 2.8 yr at pH 7 was reported
(Roy, 1972). The reported rate constant for the reaction of methacrylonitrile with ozone in the gas
phase is 2.91 x 10-18 cm3 mol/sec (Munshi et al., 1989a).
хранилище
Methyl acrylate is stored in a flammable materials storage room or cabinet below 20°C (68°F), separated from oxidizing substances. It is inhibited with 200 ppm ofhydroquinone monomethyl ether to preventself-polymerization. It is shipped in bottles,cans, drums, or tank cars.
Перевозки
UN1919 Methyl acrylate, stabilized, Hazard
Class: 3; Labels: 3-Flammable liquid.
Методы очистки
Wash the ester repeatedly with aqueous NaOH until free from inhibitors (such as hydroquinone), then wash it with distilled water, dry (CaCl2) and fractionally distil it under reduced pressure in an all-glass apparatus. Seal it under nitrogen and store it at 0o in the dark. [Bamford & Han J Chem Soc, Faraday Trans 1 78 855 1982, Beilstein 2 IV 1457.]
Несовместимости
Forms explosive mixture in air.
Incompatible with nitrates, oxidizers, such as peroxides,
strong alkalis. Polymerizes easily from heat, light, peroxides; usually contains an inhibitor, such as hydroquinone.
Утилизация отходов
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal
МЕТИЛАКРИЛАТ препаратная продукция и сырье
сырьё
препарат