1-ХЛОРО-3-НИТРОБЕНЗОЛ химические свойства, назначение, производство
Химические свойства
Moist tan or pale yellow crystalline solid
Использование
Intermediate for dyes. 1-Chloro-3-nitrobenzene was used in the preparation of aromatic azo compounds using gold (Au) nanoparticles supported on TiO2 as a catalyst. It was also used as surrogate standard to evaluate the removal of 2,4-dinitrotoluene from contaminated soils using enhanced electrokinetic remediation.
Методы производства
m-Chloronitrobenzene is of lesser economic importance
than its ortho- and para-isomers, with no U.S. production reported. The annual production in Germany was
reported to be on the order of 1000–3000 metric tons. It
has limited use in the manufacturing of dyes and agricultural
chemicals.
There is no information on potential exposure. Chloronitrobenzene
has been detected in the surface water of the
Rhine River with concentrations between 20 and 500 ng/L
and in fish at levels of up to 1 mg/kg.
Общее описание
Pale yellow crystals. Insoluble in water.
Реакции воздуха и воды
Insoluble in water.
Профиль реактивности
3-Nitrochlorobenzene can react with oxidizing materials. .
Опасность
Toxic by inhalation and ingestion. Combustible. Questionable carcinogen.
Пожароопасность
Flash point data for 3-Nitrochlorobenzene are not available. 3-Nitrochlorobenzene is probably combustible.
Профиль безопасности
Poison by ingestion and
inhalation. It forms methemoglobin in the
body and gves rise to cyanosis and blood
changes. Its effects are cumulative and
analogous to those of nitrobenzene. The
para compound is thought to be somewhat
less toxic than the ortho compound.
Chemically, it is probably converted in the
body to chloroaniline, whch is also
poisonous. In industry, it is the dust of hs
material that is most often the source of
intoxication. Flammable liquid and
dangerous fire hazard when exposed to heat
or flame. It can react with oxidizing
materials. When heated to decomposition it
emits toxic fumes of Cl-, NOx, and
phosgene. See also other
chloronitrobenzene entries and NITRO
COMPOUNDS of AROMATIC
HYDROCARBONS.
Синтез
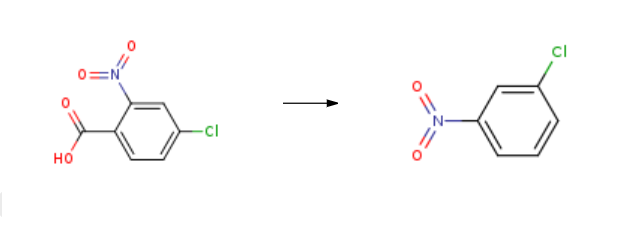
1-Chloro-3-nitrobenzene was synthesised from 4-chloro-2-nitro-benzoic acid by adding copper iodide, triethylamine and dimethyl sulfoxide in the presence of inert gas using the Schlenk technique.
Методы очистки
Crystallise the nitrobenzene from MeOH or 95% EtOH (charcoal), then pentane. [Beilstein 5 IV 722.]
1-ХЛОРО-3-НИТРОБЕНЗОЛ препаратная продукция и сырье
сырьё
препарат