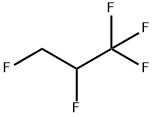
1,1,1,2,3-PENTAFLUOROPROPANE synthesis
- Product Name:1,1,1,2,3-PENTAFLUOROPROPANE
- CAS Number:431-31-2
- Molecular formula:C3H3F5
- Molecular Weight:134.05
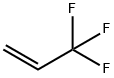
677-21-4
138 suppliers
$35.00/25g

431-31-2
20 suppliers
$155.00/5G
Yield:431-31-2 91.3 %Chromat.
Reaction Conditions:
with cobalt(II) fuoride;fluorine at 250;Inert atmosphere;Large scale;Concentration;Temperature;
Steps:
12 Experimental Examples 10 to 13
A total of 48 kg of CoF2 was added into the reactor and the regenerator, the angle of the blades attached to the paddles was adjusted, and the number of rotations of the screws of each conveyor was adjusted so that a transfer rate of CoF2 reached 48 kg/hr. Thereafter, internal temperatures of the reactor and the regenerator were increased by allowing nitrogen to flow in the reactor and the regenerator (flow rate of 2 L/min to 15 L/min), thereby removing moisture in CoF2. When the moisture in CoF2 was completely removed, the internal temperatures of the reactor and the regenerator were maintained respectively to a temperature of 250 °C while gradually decreasing a flow rate of nitrogen to 2 L/min. First, a fluorine gas was added to the regenerator at a rate of 0.94 kg/hr for 3 hours, and the addition of the fluorine gas was then suspended. A catalyst was rotated for 1 hour, the fluorine gas was added again at a rate of 0.94 kg/hr for 30 minutes, and the source gas, HFO-1243zf (CF3CH=CH2), was added to the reactor at a rate of 2.45 kg/hr. A fluorination temperature of the regenerator was constantly maintained at 250 °C. An initial molar ratio between CoF3 and CoF2 was adjusted by changing an addition time (3 hours, 2 hours and 1 hour) of an initial fluorine gas without adding the source gas (1243zf) when CoF2 was filled to 100%. When the change in initial molar ratio between CoF3 and CoF2 (3/7-->2/8-->1/9) and the change in operation conditions were performed, HFP (CF3CF=CF3) was added to completely convert CoF3 into CoF2 and a reaction system was purged with nitrogen, and thus the changes were carried out under a condition in which CoF2 was filled to 100% at the beginning. [0096] Table 2 lists ratios of products at a cobalt fluoride flow rate of 48 kg/hr according to changes in reaction temperature of the reactor and composition ratio of initial CoF3/CoF2, as analyzed using GC. As listed in Table 2, it could be seen that a ratio of the produced 1,1,1,2,3-pentafluoropropane (HFC-245eb) was increased at the same reaction temperature with a decrease in molar ratio between CoF3 and CoF2. Also, it could be seen that a ratio of the produced HFC-245eb was increased at the same molar ratio between CoF3 and CoF2 with an increase in reaction temperature. Experimental Examples 10 to 13 were carried out in the same manner as in Experimental Examples 1 to 9, except that a total of 96 kg of CoF2 was added to each of the reactor and the regenerator. [0099] The following Table 3 lists ratios of products at a cobalt fluoride flow rate of 96 kg/hr according to changes in reaction temperature of the reactor and composition ratio of initial CoF3/CoF2, as analyzed using GC. As listed in Table 3, it could be seen that the 245eb had a high yield of 80% or more, which exceeded those of Experimental Examples 1 to 9, at all the reaction temperatures compared with those listed in Table 2 (1 mole of a source gas: 10 moles of CoF3/CoF2-containing cobalt fluoride). Also, the entire amount of the catalyst was decreased, based on 1 mole of the source gas (1243zf), but the molar ratio between CoF3 and CoF2 was decreased, compared with those of Experimental Examples 1 to 9. As a result, it could be seen that the selectivity to 245eb was shown to be very excellent.
References:
EP2733135,2014,A1 Location in patent:Paragraph 0095-0099

2804-49-1
11 suppliers
$94.00/5g

431-31-2
20 suppliers
$155.00/5G
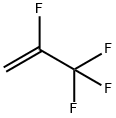
754-12-1
81 suppliers
inquiry
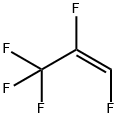
5595-10-8
9 suppliers
inquiry
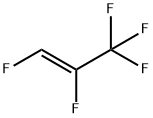
5528-43-8
12 suppliers
inquiry
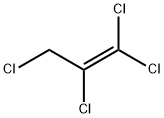
10436-39-2
33 suppliers
$155.00/1g

431-31-2
20 suppliers
$155.00/5G
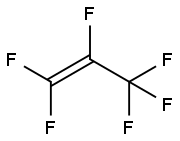
116-15-4
173 suppliers
$40.00/25g

431-31-2
20 suppliers
$155.00/5G
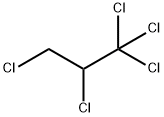
21700-31-2
16 suppliers
$60.00/500mg

431-31-2
20 suppliers
$155.00/5G