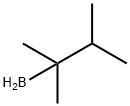
1,1,2-Trimethylpropylborane synthesis
- Product Name:1,1,2-Trimethylpropylborane
- CAS Number:3688-24-2
- Molecular formula:C6H15B
- Molecular Weight:97.99
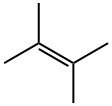
563-79-1
141 suppliers
$29.00/5mL
14044-65-6
262 suppliers
$28.50/25ml

3688-24-2
2 suppliers
inquiry
Yield:-
Reaction Conditions:
in tetrahydrofuran at 0; for 1 h;
Steps:
4
A lO mL round-bottomed flask was charged with 5 -carbon linked dimer alcohol (57 mg, 0.0885 mmol) in 1 mL of THF. To the reaction mixture was added thexylborane (0.8 mL, 3 eq.) 0.33M in THF solution obtained from BH3THF (leq) and 2,3-dimethyl-2-butene (2eq) at 0 0C for 1 hr. The reaction mixture was stirred for 2 hr and then added 3 N NaOH (0.15 mL, 5 eq) and 30% H2O2 (0.15 mL, 5 eq). The reaction mixture was warmed to room temperature and then stirred for 12 hr. The reaction mixture was quenched with H2O, extracted with EtOAc, dried over MgSθ4 and concentrated. The crude product was purified by column chromatography (EtOAc/Hexane = 3/1) to give 39.2 mg in 65% yield.
References:
THE JOHNS HOPKINS UNIVERSITY;POSNER, Gary, H.;WOODARD, Lauren, E.;LEVINE, David, R.;MOON, Deuk Kyu;MOTT, Bryan, T. WO2010/135427, 2010, A2 Location in patent:Page/Page column 65

563-79-1
141 suppliers
$29.00/5mL

3688-24-2
2 suppliers
inquiry