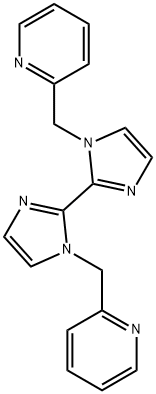
1,1-bis(pyridin-2-ylmethyl)-2,2-bisimidazole synthesis
- Product Name:1,1-bis(pyridin-2-ylmethyl)-2,2-bisimidazole
- CAS Number:1616685-69-8
- Molecular formula:C18H16N6
- Molecular Weight:316.36

492-98-8
126 suppliers
$6.00/250mg
6959-47-3
351 suppliers
$7.00/10g

1616685-69-8
7 suppliers
inquiry
Yield:1616685-69-8 40%
Reaction Conditions:
Stage #1: 2,2'-biimidazolewith sodium hydroxide in acetonitrile at 20; for 2 h;
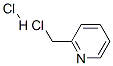
Stage #2: 2-chloromethylpyridine hydrochloride in acetonitrile at 50 - 60; for 24 h;
Steps:
3 Synthesis of 1,1'-bis(pyridin-2-ylmethyl)-2,2'-biimidazole, A3
2,2'-Biimidazole (0.27 g, 2 mmol) and NaOH (0.32 g, 8 mmol) were placed in a 100 mL round bottomed flask with 20 mL of acetonitrile. The mixture was stirred at room temperature for two hours. 2-Picolyl chloride hydrochloride (0.65 g, 4 mmol) in acetonitrile (20 mL) was added to the mixture and refluxed for 24 h at 50-60 °C. The reaction was monitored with TLC and upon completion the solvent was removed by rotary evaporation. The residue was dissolved in water (50 mL) and extracted with methylene chloride (30 mL * 3). Organic layers were combined, dried over anhydrous MgSO4 and rotary evaporated to obtain the pale brown color powder as the product. Yield: 0.25 g (40%); mp 180-183 °C; 1H NMR (δH; CDCl3, 400 MHz): 8.53 (d, 2H), 7.53 (t, 2H), 7.15 (t, 2H), 7.12 (s, 2H), 7.07 (s, 2H), 7.05 (d, 2H), 5.87 (s, 4H).
References:
Aaker?y, Christer B.;Wijethunga, Tharanga K.;Desper, John [Journal of Molecular Structure,2014,vol. 1072,# 1,p. 20 - 27]