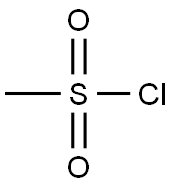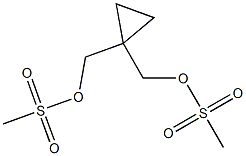
1,1-CyclopropanediMethanol diMethanesulfonate synthesis
- Product Name:1,1-CyclopropanediMethanol diMethanesulfonate
- CAS Number:136476-38-5
- Molecular formula:C7H14O6S2
- Molecular Weight:258.31
Yield:136476-38-5 86%
Reaction Conditions:
with triethylamine in propan-2-one at 0 - 20; for 3 h;
Steps:
2
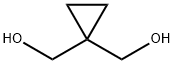
2. Alternative Procedure [0148] 1,1-Bis(hydroxymethyl)cyclopropane (40.00 g, 388 mmol) was added to a flask followed by acetone (400 mL, 15 vol) and the reaction was cooled to 0° C. Triethylamine (87.16 g, 861 mmol, 2.2 eq.) was added to the reaction and then methanesulfonyl chloride (98.68 g, 861 mmol, 2.2 eq) was added slowly such that the internal temperature does not rise above 10° C. A white precipitate forms during the addition of methanesulfonyl chloride. After the addition is complete, the reaction was allowed to stir at 0° C. for 1 h and then warmed to 20° C. and allowed to stir for 2 hours. [0149] Once the reaction was judged complete, 800 mL (30 volumes) water were added and the reaction was stirred for 15 minutes. The reaction was then filtered and washed with 100 mL water. The product was isolated on the filter as a white solid. Upon drying under vacuum at 20° C., yield 85.5 g, 86%. 1H NMR (400 MHz, CDCl3) δ 4.16 (s, 2H), 3.06 (s, 3H), 0.83 (s, 2H).
References:
US2013/324740,2013,A1 Location in patent:Paragraph 0147; 0148; 0149
39590-81-3
290 suppliers
$10.00/5g
7143-01-3
269 suppliers
$19.00/5g

136476-38-5
15 suppliers
inquiry
6914-71-2
213 suppliers
$35.00/25g

136476-38-5
15 suppliers
inquiry
598-10-7
362 suppliers
$7.00/1g

136476-38-5
15 suppliers
inquiry