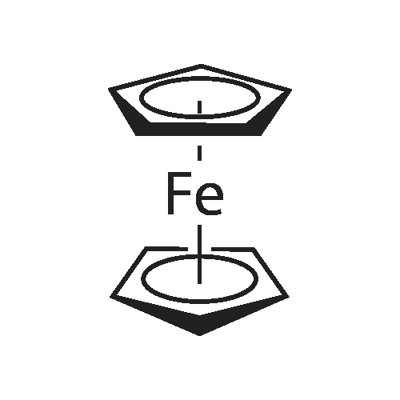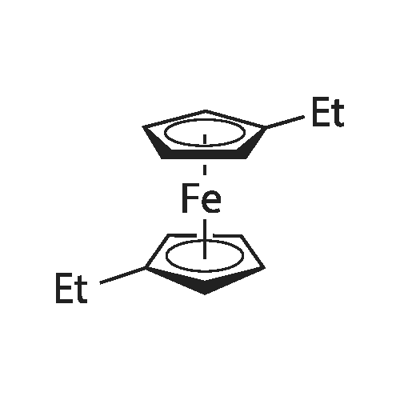
1,1'-DIETHYLFERROCENE synthesis
- Product Name:1,1'-DIETHYLFERROCENE
- CAS Number:1273-97-8
- Molecular formula:C14H18Fe10*
- Molecular Weight:242.14
Yield:-
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 25;Temperature;
Steps:
1; 2; 3 Example 1
Dissolve 2.4mol of anhydrous AlCl3 in 100ml of CH2Cl2 solvent, add 2.2mol of acetyl chloride dropwise and stir well.Then, a mixed solution of 1.0 mol ferrocene and 100 mL of dichloromethane was added dropwise;After the dropwise addition is completed, the reaction is performed at a temperature of 25 ° C to obtain a reaction mixed product;Dissolve the reaction mixture with distilled water,The organic phase was extracted with dichloromethane, and the lower organic phase product was separated. The upper aqueous phase was extracted with CH2Cl2 several times and combined into the product. The organic phase was dried over anhydrous MgSO4, MgSO4 was filtered off, and the organic solvent was removed by rotary evaporation to obtain a crude product. The crude product was repeatedly recrystallized using petroleum ether and water; neutral trialumina was used as the stationary phase packed chromatography column, and the repeatedly recrystallized product was placed on the stationary phase packed chromatography column, and eluted with an eluent to obtain Black-red acicular diacetyl ferrocene crystals with a purity of> 99.0%; 30 mL of CH2Cl2 was mixed with 0.001 mol of black-red acicular diacetyl ferrocene crystals; 0.006 mol of borane dimethylsulfide was added dropwise Ether complex reduction catalyst; after the addition is complete, the reaction is performed in an ice bath, and then the temperature is slowly raised; the reaction progress is monitored with a thin-layer color plate, and the reaction is stopped when one of the raw material points disappears; and then the excess ammonium chloride is added to terminate Reduction reaction; most of the solvent is removed, and the remaining liquid is washed with water multiple times. The collected organic phase is dried over anhydrous MgSO4, and the solvent is evaporated under reduced pressure to obtain a reduced crude diethylferrocene; Motoji Crude ferrocene was added with 200-300 mesh silica gel, packed in a chromatography column, and a mixed solvent of petroleum ether and ethyl acetate in a volume ratio of 20: 1 was used as the eluent to elute the column to obtain high-purity diethyl Ferrocene solution; the solvent in the obtained high-purity diethylferrocene solution was removed by a rotary evaporation method to obtain diethylferrocene having a purity of greater than 99.0%
References:
Northwestern Polytechnical University;Ma Xiaoyan;Liu Xiaoju;Suo Qi CN110551159, 2019, A Location in patent:Paragraph 0037; 0038; 0039
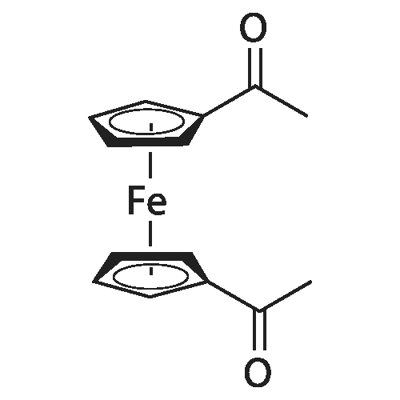
1273-94-5
140 suppliers
$10.00/1g

1273-97-8
95 suppliers
$18.00/1g

1273-94-5
140 suppliers
$10.00/1g
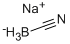
25895-60-7
530 suppliers
$9.00/1g

1273-97-8
95 suppliers
$18.00/1g

1273-94-5
140 suppliers
$10.00/1g

7732-18-5
485 suppliers
$12.69/100ml

1273-97-8
95 suppliers
$18.00/1g

1273-94-5
140 suppliers
$10.00/1g
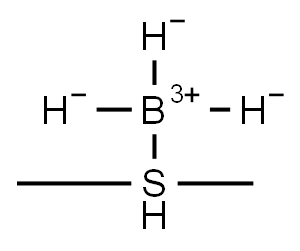
13292-87-0
250 suppliers
$18.19/5ml

1273-97-8
95 suppliers
$18.00/1g