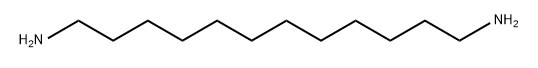
1,12-Dodecanediamine synthesis
- Product Name:1,12-Dodecanediamine
- CAS Number:2783-17-7
- Molecular formula:C12H28N2
- Molecular Weight:200.36
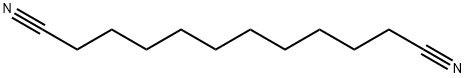
4543-66-2
49 suppliers
$63.50/5g

2783-17-7
218 suppliers
$9.00/5g
Yield:2783-17-7 91.7%
Reaction Conditions:
with hydrogen;sodium methylate in methanol at 130; under 37503.8 Torr; for 5 h;
Steps:
1 Reference Example 1 Synthesis of 1,12-diaminododecane (dodecamethylenediamine)
Thermocouple thermometer,10 g of 1,10-dicyanodecane, 29.4 g of methanol, 0.36 g of a 28 wt% sodium methoxide-methanol solution (in methanol 3.0% by weight), and 0.27 g of a nickel catalyst developed in the same manner as in Example 1 was charged. After charging, hydrogen substitution was carried out, and thereafter the pressure was increased to 5 MPa. The temperature was elevated to an internal temperature of 130 ° C. over about 1 hour after boosting pressure.The reaction started at 130 ° C., and it was confirmed that almost no hydrogen consumption was consumed in about 3 hours. After further reacting for 1 hour, cooling down to room temperature, depressurizing, filtering the reaction solution to remove the catalyst, and washing the filtrate with 100 g of methanol. Methanol concentration was performed from the resultant methanol reaction solution of 1,12-diaminododecane. As a result, the concentration of crude 1,12-diaminododecane obtained after concentration was 10.34 g. The GC area% of 1,12-diaminododecane was 96.8 area%, and the reaction yield of 1,12-diaminododecane calculated from the quantitative value was 91.7%.
References:
JP2017/31143,2017,A Location in patent:Paragraph 0091-0094

38279-34-4
5 suppliers
inquiry

2783-17-7
218 suppliers
$9.00/5g
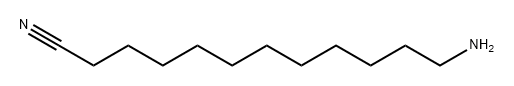
26437-41-2
1 suppliers
inquiry

2783-17-7
218 suppliers
$9.00/5g
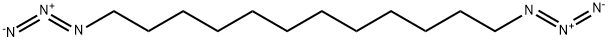
113665-32-0
0 suppliers
inquiry

2783-17-7
218 suppliers
$9.00/5g
5675-51-4
290 suppliers
$5.00/10g

2783-17-7
218 suppliers
$9.00/5g