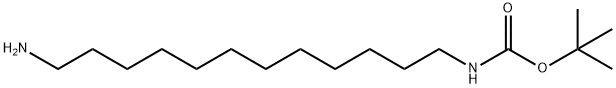
1-BOC-1,12-DIAMINODODECANE synthesis
- Product Name:1-BOC-1,12-DIAMINODODECANE
- CAS Number:109792-60-1
- Molecular formula:C17H36N2O2
- Molecular Weight:300.48
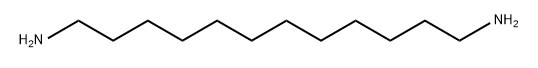
2783-17-7
218 suppliers
$9.00/5g

24424-99-5
857 suppliers
$13.50/25G

109792-60-1
58 suppliers
$61.00/100mg
Yield: 99%
Reaction Conditions:
with sodium hydroxide in dichloromethane;water for 1.08333 h;
Steps:
48 Example 48
Mono Protection of 1,12-Diaminododecane with BOC.
;1,12-Diaminododecane (1.0 g, 10 mmol amine, Aldrich Chemical Company) was taken up with dichloromethane (25 ML) and H2O (25 ML).sodium hydroxide (2 pellets, Fisher Scientific) were added and the reaction was stirred vigorously. Di-tert-butyl dicarbonate (500 mg, 2.3 mmol, Aldrich Chemical Company) was taken up in dichloromethane (10 ML) and added dropwise to the stirring reaction over 5 min.After 1 hr, the reaction was analyzed by TLC and mass spectrometry (Sciex API 150EX) to verify that the reaction was complete.The reaction was transferred to a separatory funnel and the layers were separated.The organic layer was concentrated under reduced pressure.Half of the concentrated material was brought up in hot acetonitrile (100 ML), and crystallized.The material was spun down by centrifugation and washed with acetonitrile (2*25 ML).The material was dried on high vacuum and yielded crystalline material (745 mg, 99% yield).The product, boc-aminedodecaneamine was verified by TLC and mass spectrometry (Sciex API 150EX).
References:
Monahan, Sean D.;Nader, Lisa;Wolff, Jon A.;Budker, Vladimir G.;Hagstrom, James E. US2003/235916, 2003, A1 Location in patent:Page 21

2783-17-7
218 suppliers
$9.00/5g
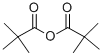
1538-75-6
220 suppliers
$17.67/10gm:

109792-60-1
58 suppliers
$61.00/100mg

2783-17-7
218 suppliers
$9.00/5g
58632-95-4
275 suppliers
$7.00/5g

109792-60-1
58 suppliers
$61.00/100mg