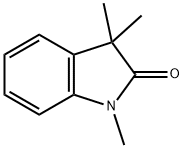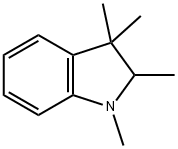
1,2,3,3-Tetramethyl-2,3-dihydro-1H-indole synthesis
- Product Name:1,2,3,3-Tetramethyl-2,3-dihydro-1H-indole
- CAS Number:13034-76-9
- Molecular formula:C12H17N
- Molecular Weight:175.27
118-12-7
195 suppliers
$5.00/5g

13034-76-9
2 suppliers
inquiry
Yield:13034-76-9 100%
Reaction Conditions:
with [P(H)t-Bu2][B(C6F5)4];1,3-diisopropylimidazol-2-ylidene-9-borabicyclo[3.3.1]nonane;hydrogen in chlorobenzene at 100; under 77525.2 Torr; for 4 h;Glovebox;Time;Temperature;Reagent/catalyst;
Steps:
3
General procedure: [001141 Reaction 2[001151 In a glovebox IiPr2-BBN(3.9 mg, 0.016 mmol, 1 eq), [tBu3PHj[B(C6F5)4j (14.0 mg, 0.0159 mmol, 1 eq) and N-benzylidene-tert-butylamine (255.4 mg, 1.584 mmol, 100 eq.) were weighed into vials. N-benzylidene-tert-butylamine and IiPr2-BBN were transferred to the vial containing [tBu3PHj[B(C6F5)4j using 0.6 mL toluene. The vial was equipped with a stir bar and placed in a Parr pressure reactor. The reactor was sealed, removed from the glovebox and attached to a thoroughly purged hydrogen gas line. The reactor was purged ten times at 50 atm hydrogen and ten times at 102 atm hydrogen. The reactor was sealed under 102 atm hydrogen and placed on a stir plate for 2 hours at room temperature. The reactor was then slowly vented and the sample was concentrated in vacuo. Conversion of N-benzylidene-tert-butylamine to N-benzyl-tert-butylamine was determined by ‘H-NMR in toluene-d8.
References:
WO2013/177708,2013,A1 Location in patent:Paragraph 0020; 0021
5418-63-3
117 suppliers
$6.00/1g

13034-76-9
2 suppliers
inquiry

1640-39-7
331 suppliers
$6.00/5g

13034-76-9
2 suppliers
inquiry

5418-63-3
117 suppliers
$6.00/1g

1640-39-7
331 suppliers
$6.00/5g
1971-46-6
0 suppliers
inquiry

13034-76-9
2 suppliers
inquiry

74-88-4
351 suppliers
$15.00/10g