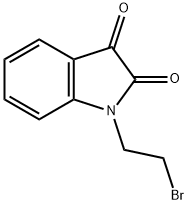
1-(2-BROMOETHYL)-1H-INDOLE-2,3-DIONE synthesis
- Product Name:1-(2-BROMOETHYL)-1H-INDOLE-2,3-DIONE
- CAS Number:4290-78-2
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
91-56-5
440 suppliers
$5.00/25g

106-93-4
376 suppliers
$15.00/5g

4290-78-2
12 suppliers
$106.00/25mg
Yield:4290-78-2 89%
Reaction Conditions:
Stage #1: indole-2,3-dionewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.166667 h;
Stage #2: ethylene dibromide in N,N-dimethyl-formamide at 80; for 0.166667 h;Microwave irradiation;
Steps:
Synthesis of Isatin Derivatives (1b-1f)
General procedure: A mixture ofisatin (5mmol) and potassium carbonate (8mmol) in DMF(10 mL) was stirred for 10minutes at roomtemperature. Alkylhalides (6mmol; benzyl bromide for preparation of 1c, 1d,and 1e; CH3I forpreparationof 1b; and1,3-dibromoethane forpreparation of 1f) were added dropwise to the reaction mixtureand then the reaction was microwave irradiated usinga multimode reactor (Synthos 3000, Anton Paar GmbH,Graz, Austria) (1,400W maximum magnetron). The vesselswere heated for 5 minutes at 80°C and held at the sametemperature for a further 5 minutes (400 W). Cooling wasaccomplished by a fan (5 minutes). The final product wasdried and recrystallized from ethanol. All the spectral datafor the products obtained were in good agreement with thereported data.
References:
El-Faham, Ayman;Hozzein, Wael N.;Wadaan, Mohammad A. M.;Khattab, Sherine N.;Ghabbour, Hazem A.;Fun, Hoong-Kun;Siddiqui, Mohammed Rafiq [Journal of Chemistry,2015,vol. 2015,art. no. 716987]

62-53-3
636 suppliers
$10.00/1g

4290-78-2
12 suppliers
$106.00/25mg
