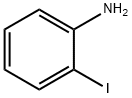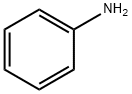
Aniline synthesis
- Product Name:Aniline
- CAS Number:62-53-3
- Molecular formula:C6H7N
- Molecular Weight:93.13
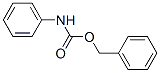
3422-02-4
3 suppliers
inquiry

62-53-3
688 suppliers
$10.00/1g
Yield:62-53-3 95%
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen;sodium hydroxide in methanol at 20; under 750.075 Torr; for 2 h;Autoclave;Green chemistry;
Steps:
11 Example 11
In an autoclave, 220 g (1 mol) of N-Cbz-aniline was added to 1300 mL of methanol.Then add 1000 mL of saturated sodium hydroxide solution, and then add 22 g of palladium carbon in a content of 5%, and replace the gas in the reaction flask three times with nitrogen.Then replace it twice with hydrogen, pass hydrogen gas to make the pressure in the autoclave reach 0.1 MPa, and react at room temperature for 2 h. TLC monitors the reaction of the starting material completely and filters the reaction solution.Further, 50 g of activated carbon was added to the filtrate, stirred for 20 minutes, filtered, and the filtrate was concentrated to obtain 90 g of aniline in a yield of 95%.
References:
CN108440409,2018,A Location in patent:Paragraph 0041; 0042
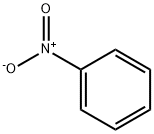
98-95-3
34 suppliers
$14.00/25g

62-53-3
688 suppliers
$10.00/1g
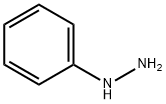
100-63-0
382 suppliers
$10.00/1g

62-53-3
688 suppliers
$10.00/1g
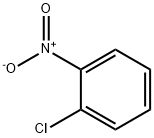
88-73-3
49 suppliers
$14.00/25g

62-53-3
688 suppliers
$10.00/1g