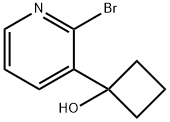
1-(2-Bromopyridin-3-yl)cyclobutanol synthesis
- Product Name:1-(2-Bromopyridin-3-yl)cyclobutanol
- CAS Number:780801-06-1
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
Yield:780801-06-1 56%
Reaction Conditions:
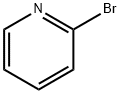
Stage #1: 2-bromo-pyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 1.83333 h;Inert atmosphere;
Stage #2: cyclobutanonewith water;sodium hydrogencarbonate in tetrahydrofuran;hexane at -78 - 20; for 1.33333 h;Inert atmosphere;
Steps:
Representative procedure for the preparation of 2:
General procedure: 1-(2-bromopyridin-3-yl)cyclopentanol (2a). To a cooled (-78°) solution of diisopropylamine (18.3 g,25.4 mL, 0.181 mol) in absolute THF (700 mL), n-BuLi (69 mL, 23% in hexane,2.5 M, 0.172 mol) was added dropwise under argon atmosphere. The mixture was stirred at -78oC for 1 h, and a solution of 2-bromopyridine (23.1 g, 14.3 mL, 0.146mmol) in dry THF (150 mL) was added dropwise over 20 min. The reaction mixture was kept at -78° for 90 min, and pre-cooled (-78°) solution of cyclopentanone (26.6 g, 28 mL, 0.316 mol) in dry THF (70 mL) was added in one portion. The mixture was stirred at -78° for additional 80 min and quenched with saturated aq NaHCO3 (140 mL) at -78°, then warmed to rt and diluted with EtOAc (1 L). The organic phase was separated, washed with saturated aq NaHCO3(2×150 mL) and brine (200 mL), dried over Na2SO4, and concentrated in vacuo togive 22.5 g of residue. The product was purified by flash chromatography (gradient hexane - EtOAc as an eluent), followed by recrystallization from pentane. The sample of 2a of analytical purity was obtained by additional recrystallization from hexanes. Yield 14.2 g (40%).
References:
Subota, Andrii I.;Grygorenko, Oleksandr O.;Valter, Yevheniia B.;Tairov, Maxim A.;Artamonov, Oleksiy S.;Volochnyuk, Dmitriy M.;Ryabukhin, Sergey V. [Synlett,2015,vol. 26,# 3,art. no. ST-2014-D0832-L,p. 408 - 411] Location in patent:supporting information

1191-95-3
322 suppliers
$10.00/1g

780801-06-1
5 suppliers
inquiry