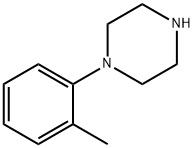
1-(2-Methylphenyl)piperazine synthesis
- Product Name:1-(2-Methylphenyl)piperazine
- CAS Number:39512-51-1
- Molecular formula:C11H16N2
- Molecular Weight:176.26
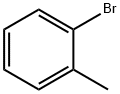
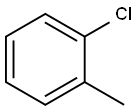
95-46-5
291 suppliers
$10.00/10 g

57260-71-6
740 suppliers
$5.00/5g

39512-51-1
120 suppliers
$15.00/1g
Yield: 82%
Reaction Conditions:
Stage #1:2-methylphenyl bromide;1-t-Butoxycarbonylpiperazine with methanesulfonato(2-dicyclohexylphosphino-2’,6’-di-i-propoxy-1,1’-biphenyl)(2’-methylamino-1,1‘-biphenyl-2-yl)palladium(II);sodium t-butanolate in 1,4-dioxane at 100;
Stage #2: with trifluoroacetic acid in dichloromethane at 20;
Steps:
General Procedure 1
General procedure: A mixture of 1-BOC-piperazine (1 equiv.), sodium tert-butoxide (1.5 - 3 equiv.), arylhalide (1.2 equiv.) and Pd-Ruphos G3 (0.1 equiv.) were dissolved in pre-degassed 1,4-dioxane (0.15 - 0.3 M). The reaction mixture was then heated at 100 °C until complete. The reaction mixture was cooled to ambient temperature and then either treated with an aqueous work up or with filtration through celite. The solvent was dried (using either MgSO4, Na2SO4 or a phase separator) and subsequently removed under vacuum. The residue was purified by flash silica chromatography to give the product or used directly in the next step. The product was then dissolved in DCM (4-5 mL) and TFA (0.5 - 1 ml) added (either at 0 °C or at room temperature). The reaction mixture was stirred at ambient temperature until complete (LCMS monitoring). Then, the reaction was filtered through an SCX cartridge eluting first with methanol then with ammonia (7 M, 4 M or 2 M) in methanol. The ammonia in methanol solution was concentrated under reduced pressure to give the desired product.
References:
UCB Biopharma SPRL;The designation of the inventor has not yet been filed EP3527209, 2019, A1 Location in patent:Paragraph 0064-0065; 0088-0089
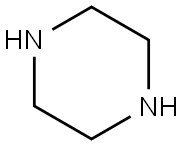
110-85-0
552 suppliers
$5.00/5 g

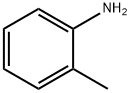
95-46-5
291 suppliers
$10.00/10 g

39512-51-1
120 suppliers
$15.00/1g
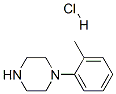
95356-15-3
36 suppliers
$45.00/50mg

39512-51-1
120 suppliers
$15.00/1g