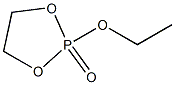
1,3,2-Dioxaphospholane, 2-ethoxy-, 2-oxide synthesis
- Product Name:1,3,2-Dioxaphospholane, 2-ethoxy-, 2-oxide
- CAS Number:823-31-4
- Molecular formula:C4H9O4P
- Molecular Weight:152.0856
Yield: 95%
Reaction Conditions:
in dichloromethane at 0 - 20; for 12 h;
Steps:
22 Example 22Preparation of compound 22 (ethoxy vinyl phosphate or 2-ethoxy-2-oxo-1,3,2-dioxaphospholane
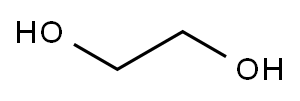
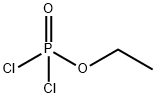
Add 200 g of dichloromethane and 51 g of ethanol (reactant C) to a 500 ml three-necked flask at room temperature and stir for 0.5 h at room temperature. The solution is colorless and transparent. The three-necked flask is connected to an external gas absorption device, and then 143 g of the reaction intermediate B is dropped through The liquid funnel was slowly added to a 500ml three-necked flask, the dropping rate was controlled to 1 drop / second, and the reaction temperature was 0 ° C. With the addition of the reaction intermediate B, a large amount of heat was released and a large amount of HCl gas was released. The solution was colorless and transparent. Stirring was continued at room temperature for 12 hours, and then dichloromethane was removed to obtain a light yellow liquid. The light yellow liquid was distilled under reduced pressure at 120 ° C. to obtain 144 g of a colorless transparent liquid with a yield of 95%. This product is compound 22 (ethoxy vinyl phosphate or 2-ethoxy-2-oxo-1,3,2-dioxocyclopentane). The HCl gas generated by the reaction is absorbed by water to obtain hydrochloric acid. The hydrochloric acid is sold as a chemical raw material. The organic solvent used in the reaction is distilled and dried to be recovered and reused.
References:
Heng Da New Energies Science And Technology Group Co., Ltd.;Shi Yinghua;Zhao Wenwen;Zhang Qing;Han Wenyu CN110590848, 2019, A Location in patent:Paragraph 0150-0152
695-11-4
3 suppliers
inquiry

823-31-4
8 suppliers
inquiry

64-17-5
706 suppliers
$10.00/50g
6609-64-9
208 suppliers
$34.93/5G

6711-47-3
0 suppliers
inquiry

823-31-4
8 suppliers
inquiry