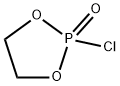
2-Chloro-1,3,2-dioxaphospholane-2-oxide synthesis
- Product Name:2-Chloro-1,3,2-dioxaphospholane-2-oxide
- CAS Number:6609-64-9
- Molecular formula:C2H4ClO3P
- Molecular Weight:142.48
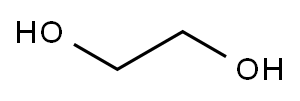
107-21-1
1327 suppliers
$10.00/25g

6609-64-9
210 suppliers
$34.93/5G
Yield:6609-64-9 92%
Reaction Conditions:
with trichlorophosphate in dichloromethane at 0; for 14 h;
Steps:
S01-S02 Preparation of reaction intermediate B:
S01: At room temperature, add 310 g of ethylene glycol (reactant A) and 350 g of anhydrous dichloromethane (non-aqueous solvent) to a three-necked flask with a capacity of 2000 mL, and then reduce the temperature to 0 ° C, and continue stirring for 0.5 h until the solution appears Colorless and transparent, three-necked bottle connected with external gas absorption device.In step S01, the non-aqueous solvent is added to make the reactants more uniformly dispersed and provide a stable reaction environment. The non-aqueous solvent treatment in this step uses methylene chloride, and acetonitrile, tetrahydrofuran, chloroform, ethyl acetate, dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate, n-hexane, cyclohexane, Benzene and toluene are mixtures of one or more of these organic solvents with good dispersibility and no effect on the reaction process. The added mass of the non-aqueous solvent can be set according to the specific state of the reaction system so that it can meet the requirements of the reaction process. Generally, the added mass is 1-6 times the mass of the reactant A. The reaction in the reaction system The larger the viscosity, the more difficult it is to disperse, and the amount of non-aqueous solvent often increases correspondingly.S02: 770 g of phosphorus oxychloride dissolved in 300 g of anhydrous dichloromethane is slowly added to a three-necked bottle through a dropping funnel, and the dropping rate is controlled to 1 drop / second. In the case that the total mass of the reaction system is small, phosphorus oxychloride can be directly added dropwise. In this embodiment, since the overall volume of the reaction system is large, the phosphorus oxychloride is first diluted and then added dropwise so that The reaction can be more complete, and there is no special requirement for the amount of the diluted solvent, and it can be adjusted according to the total amount of the reaction system and the demand during the reaction. The diluent used here is the same as the non-aqueous solvent in S01, both are dichloromethane, which is conducive to the subsequent process steps of removing the non-aqueous solvent by atmospheric distillation (no need to change the conditions, only a single solvent can be processed).With the continuous addition of the dichloromethane solution of phosphorus oxychloride, the reaction emits a lot of heat and a large amount of HCl gas is released. The reaction temperature is maintained at 0 ° C. After the dichloromethane solution of phosphorus oxychloride is completely dripped, it continues. The reaction system was stirred until it was colorless and transparent (2h), and then the reaction was continued to be stirred for 12h until no gas was generated. Then, the reaction system was distilled under normal pressure to remove dichloromethane to obtain a pale yellow liquid, that is, a crude product of the reaction intermediate B. The crude product of the reaction intermediate B was distilled under reduced pressure at 80 ° C to obtain 656 g of a colorless transparent liquid with a yield of 92%; the product was the reaction intermediate B (vinyl chlorophosphate or 2-chloro-2-oxo-1, 3,2-dioxolane).
References:
Heng Da New Energies Science And Technology Group Co., Ltd.;Shi Yinghua;Zhao Wenwen;Zhang Qing;Han Wenyu CN110590848, 2019, A Location in patent:Paragraph 0053-0062
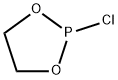
822-39-9
104 suppliers
$16.80/1G

6609-64-9
210 suppliers
$34.93/5G

7381-30-8
213 suppliers
$10.00/250mg

6609-64-9
210 suppliers
$34.93/5G