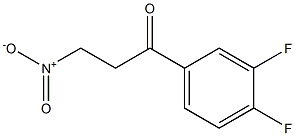
1-(3,4-Difluorophenyl)-3-nitro-1-propanone synthesis
- Product Name:1-(3,4-Difluorophenyl)-3-nitro-1-propanone
- CAS Number:1345413-22-0
- Molecular formula:C9H7F2NO3
- Molecular Weight:215.1536
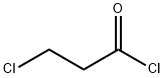
1215969-79-1
3 suppliers
inquiry

1345413-22-0
4 suppliers
inquiry
Yield:1345413-22-0 78.125%
Reaction Conditions:
with 3,5-dihydroxyphenol;sodium iodide;sodium nitrite in N,N-dimethyl-formamide at 5 - 30;Product distribution / selectivity;Inert atmosphere;
Steps:
6
Example 6; Preparation of l-(3',4'-difluorophenyl)-3-nitro-propan-l-one; [0110] 3-Chloro-l-(3', 4'-difluorophenyl)-propan-l-one (700 g) and N,N- dimethylformamide (1400 ml) were taken into a reaction assembly under nitrogen atmosphere, followed by cooling the mass to 5-10°C. Phloroglucinol (154 g) and sodium iodide (7.0 g) were added to the resulting suspension while maintaining the temperature at 5- 10°C. Sodium nitrite (472.5 g) was added to the resulting mass while maintaining the temperature between 5-10°C. The resulting reaction mass was stirred for 30 minutes at 5- 10°C, followed by raising the mass temperature to 25-30°C and maintaining for 3 to 4 hours. Toluene (3500 ml) and water (3500 ml) were added into the reaction mass obtained after completion of the reaction, followed by stirring for 15 minutes. The layers were separated and the aqueous layer was extracted with toluene (2 x 1750 ml). The toluene layers were combined and washed with water (3 x 2100 ml). The resulting toluene layer was filtered though hyflo supercel and the bed was washed with toluene (2 x 350 ml). The main filtrate and the washing were combined and concentrated to dryness while maintaining the temperature at 50°C under reduced pressure, followed by isopropyl alcohol (2 x 350 ml) stripping. The concentrated mass was dissolved in isopropyl alcohol (2100 ml) at 50-55°C. The resulting clear solution was gradually cooled to 35-45°C and then seeded with l-(3',4'- difluorophenyl)-3-nitro-propan-l-one (10.0 g) at 35-40°C. The resulting mass obtained after addition of seeding was stirred for 5 hours, followed by cooling to 20-25°C. The resulting slurry was stirred for 8 to 10 hours at 20-25°C. The resulting slurry was further cooled to -5 to 0°C, followed by stirring for 2 hours at -5 to 0°C. The product was isolated by filtration and washed with chilled isopropyl alcohol (175 and 700 ml). The wet product was dried under reduced pressure at 30-35°C till isopropyl alcohol content is less than 1000 ppm to obtain 575 g of l-(3',4'-difluorophenyl)-3-nitro-propan-l-one (Yield: 78.125%, HPLC Purity : 99.86% by area).
References:
WO2011/132083,2011,A2 Location in patent:Page/Page column 27

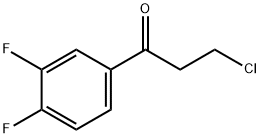
367-11-3
310 suppliers
$10.00/1g

1345413-22-0
4 suppliers
inquiry