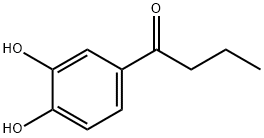
1-(3,4-Dihydroxyphenyl)butan-1-one synthesis
- Product Name:1-(3,4-Dihydroxyphenyl)butan-1-one
- CAS Number:17386-89-9
- Molecular formula:C10H12O3
- Molecular Weight:180.2
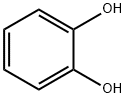
120-80-9
501 suppliers
$13.00/25g

141-75-3
337 suppliers
$12.32/25G

17386-89-9
11 suppliers
$87.00/100mg
Yield:17386-89-9 82%
Reaction Conditions:
with aluminum (III) chloride in 1,2-dichloro-ethane at 0 - 20; for 24 h;Friedel-Crafts Acylation;
Steps:
Acylation of pyrocatechol; General Procedure
General procedure: The appropriate acyl chloride (0.45 mol) was added dropwise to a stirred suspension of pyrocatechol (33 g, 0.3 mol) and aluminum chloride (120 g, 0.9 mol) in 1,2-dichloroethane (500 mL) on an ice bath during 1 h. The reaction mixture was stirred in an ice bath for 3 hours, then warmed to room temperature, stirred 20 h, quenched with cold dilute hydrochloric acid solution (5%, 1 L). The precipitate was filtered off, washed with water and dried. The organic layer was separated, washed with water, dried (Na2SO4) and evaporated. The aqueous layer was extracted with ethyl acetate (3 x 300 mL). An ethyl acetate solution was washed with water, dried (Na2SO4) and evaporated. The residues were combined with the precipitate and used in the next step without further purification. A small amount of the substance was purified by flash chromatography (silica gel, hexane/acetone, 5:1) for analytical purposes.
References:
Khmelevskaya, Ekaterina A.;Pelageev, Dmitry N. [Tetrahedron Letters,2019,vol. 60,# 15,p. 1022 - 1024] Location in patent:supporting information

120-80-9
501 suppliers
$13.00/25g

107-92-6
560 suppliers
$9.00/5g

17386-89-9
11 suppliers
$87.00/100mg
4112-92-9
7 suppliers
inquiry

17386-89-9
11 suppliers
$87.00/100mg

120-80-9
501 suppliers
$13.00/25g
98-95-3
9 suppliers
$14.00/25g

141-75-3
337 suppliers
$12.32/25G

17386-89-9
11 suppliers
$87.00/100mg