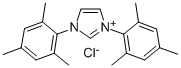
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride synthesis
- Product Name:1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
- CAS Number:141556-45-8
- Molecular formula:C21H25ClN2
- Molecular Weight:340.89

50-00-0
846 suppliers
$10.00/25g
56222-36-7
25 suppliers
$90.71/200MG

141556-45-8
224 suppliers
$8.00/250mg
Yield:141556-45-8 74.6%
Reaction Conditions:
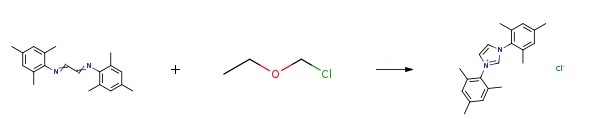
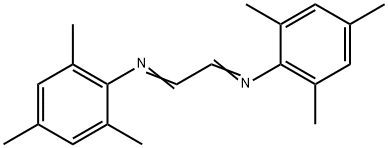
Stage #1: formalin;(1E,2E)-N1,N2-bis(2,4,6-trimethylphenyl)ethane-1,2-diimine in ethyl acetate at 75;
Stage #2: with chloro-trimethyl-silane in ethyl acetate; for 2.08333 h;
Steps:
24
(1E,2E)-N1,N2-(2,4,6-trimethylphenylethane)-1,2-diimine (2.92 g, about 10 mmol) and paraformaldehyde (0.3 g, about 10mmol) was dissolved in 10mL of ethyl acetate and added to a 100mL two-necked flask, Stir and reflux at 75°C, gradually add a solution of 5 mL of ethyl acetate containing trimethylchlorosilane (1.195 g, about 11 mmol) dropwise, add dropwise for 5 min, and react for 2 h, after cooling to room temperature, it was sealed and placed in a -20°C refrigerator for cooling for 12 hours, filtered with suction, and washed with ethyl acetate to obtain 2.5 g of a green-gray solid with a yield of 74.6% to obtain 1,3-bis(2,4,6-trimethylphenyl)imidazolium chloride.
References:
CN114644661,2022,A Location in patent:Paragraph 0208; 0211

56222-36-7
25 suppliers
$90.71/200MG
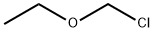
3188-13-4
134 suppliers
$35.00/5G

141556-45-8
224 suppliers
$8.00/250mg

50-00-0
846 suppliers
$10.00/25g

56222-36-7
25 suppliers
$90.71/200MG

141556-45-8
224 suppliers
$8.00/250mg

50-00-0
846 suppliers
$10.00/25g

74663-75-5
43 suppliers
$172.00/1g

141556-45-8
224 suppliers
$8.00/250mg

88-05-1
313 suppliers
$6.00/1g

141556-45-8
224 suppliers
$8.00/250mg