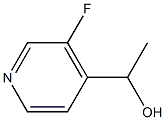
1-(3-Fluoro-pyridin-4-yl)-ethanol synthesis
- Product Name:1-(3-Fluoro-pyridin-4-yl)-ethanol
- CAS Number:87674-15-5
- Molecular formula:C7H8FNO
- Molecular Weight:141.14

75-07-0
422 suppliers
$14.00/5mL

87674-15-5
36 suppliers
$31.00/100mg
Yield: 76%
Reaction Conditions:
Stage #1:3-Fluoropyridine with lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 3.5 h;
Stage #2:acetaldehyde in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 1.5 h;
Stage #3: with water;ammonium chloride in tetrahydrofuran;n-heptane;ethylbenzene at -30;
Steps:
2
EXAMPLE 2 2-Bromo-1-(3-fluoropyridin-4-yl)ethanone hydrobromide Into a stirred solution of 3-fluoropyridine (14 g, 144.2 mmol) in anhydrous THF (150 mL), cooled to -78° C. and under argon, 79.2 mL (158.6 mmol) of a 2N solution of lithiumdiisopropylamide (LDA) in n-heptane, THF, ethylbenzene, were slowly dropped in about 1 h. After stirring for 2.5 h a cooled solution (ca. 0° C.) of acetaldehyde (8.9 mL, 158.5 mmol) in 25 mL of anhydrous THF was slowly dropped and the reaction mixture was stirred at -78° C. for 1.5 h. The solution was warmed to -30° C. and a solution of ammonium chloride (150 g) in 700 mL of water was added. The mixture was extracted with ethylacetate (3*400 mL) and the organic layers were washed with brine (4*200 mL) and dried over sodium sulfate. After concentration, the oil was crystallized with n-hexane (40 mL) and 15.6 g (76% yield) of 1-(3-fluoropyridin-4-yl)ethanol were obtained.
References:
Pharmacia Italia S.p.A. US2007/142415, 2007, A1 Location in patent:Page/Page column 17
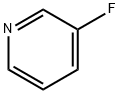
372-47-4
379 suppliers
$5.00/1g

75-07-0
422 suppliers
$14.00/5mL

87674-15-5
36 suppliers
$31.00/100mg